7 Biochemistry Lab Experiments that are Easier to Teach with Labster

You want the best biochemistry labs for your students. Maybe lesson planning is taking up too much time, you’re recycling the same labs over and over, or you’re a new teacher. Whatever the case, we’ve gathered 7 biochemistry lab experiments that you can teach your students. We’ve also matched accompanying virtual labs that can help teach some of the experiments.
- DNA/RNA Sequencing
- Gel Electrophoresis
- Antibodies & Antigens
- Blotting Methods
- Polymerase Chain Reaction (PCR)
ELISA stands for enzyme-linked immunosorbent assay. basic assay technique. Trying to capture a specific protein amongst thousands of types of proteins is like looking for a needle in a haystack, but it’s easier with this basic assay technique. Instructors can provide ELISA kits if budgets allow.
There are incubation times necessary with this method, so the collapsed time aspect of using a virtual lab is useful! Labster has an ELISA Virtual Lab where students can help a doctor quantify Factor IX protein, which is used for hemophilia drugs.

Figure alt text: Sealing an ELISA plate using a microplate seal in a virtual lab.

2.) DNA/RNA Sequencing
With the right equipment, DNA & RNA sequencing can be straightforward and easy. Fast and affordable sequencing matters, as it opens up a whole new world for biology/biochemistry research. There are a variety of ways to do sequencing, but they can be expensive - especially for a lab class.
Labster has a few sequencing labs, one being RNA Extraction: Sample and purify mRNA from pigs where students can learn how to extract RNA from pig fat tissue samples and how to purify messenger RNA using magnetic beads.
3.) Nutrition
There’s a great deal of biochemistry research done in the field of nutrition such as understanding how food relates to cancer and how food can promote health. A CUNY lab exercise guide outlines some different hands-on labs instructors can do such as “The Microbiology of Milk and Food.”
Some concepts can be difficult to get across to students in a regular lab or through lecturing. That’s where our 3D animations and interactivity are most impactful. Labster has a Carbohydrates virtual lab where students can visualize and explore how carbohydrates are broken down by the digestive system and taken up into the bloodstream.

Figure alt text: Reviewing various carbohydrates in a virtual lab.
4.) Gel Electrophoresis
This technique is used to separate components of a mixture, often DNA, RNA, or proteins. Kits are available to do this experiment in the classroom if the budget allows for it. Virtual lab simulations are a cost-effective alternative Not only are they helpful for learning the technique itself, but also to visualize the components.
In Labster’s Gel Electrophoresis virtual lab , students will solve a crime by using DNA fingerprinting to identify a thief. Students will use nucleic acid gel electrophoresis to separate and visualize DNA molecules and watch an animation to understand what happens inside the gel tank.
5.) Antibodies & Antigens
Antibodies and Antigens can be difficult to teach but Labster has a free 3D animation video on “Antigen-Antibody Binding - Why are some blood types incompatible?” Utilizing videos, interactive simulations, lectures, and images help to differentiate teaching approaches and support students in learning these concepts.
In Labster’s Antibodies virtual lab , learn about the concepts of antibodies and antigens, as well as the ABO and Rhesus blood grouping systems and their importance in blood transfusions. Then, they will help a young couple determine the potential risk for Rhesus disease in their unborn child.

Figure alt text: Tight antibody-antigen complex.
6.) Blotting Methods
Students need to use blotting methods to identify DNA, RNA, and other proteins. There are a variety of blots: Northern, Southern, Eastern, and Western (1). This technique can be done in the lab - but it is notoriously difficult at first. Practicing in a virtual lab helps students grasp the methods and understand how to analyze their results.
In our Western Blot virtual lab , students perform a western blot experiment to ultimately provide data and knowledge to identify a promising treatment for breast cancer.
7.) Polymerase Chain Reaction
Polymerase Chain Reaction (PCR) is used to amplify or make multiple copies of DNA (1). It can be expensive to run these labs as PCR labs are expensive and time-consuming. This is where virtual labs can help!
In the Polymerase Chain Reaction (PCR) simulation , students will be thrown right into a crime scene where a murder has taken place. After investigating the crime scene, their first task is to collect blood samples in the hope that the murderer has left traces of their DNA. A 3D animation will show the PCR experiment at the molecular level, illustrating the structure of DNA and its replication.

If you’re interested in teaching PCR in an approachable way, check out our PCR blog post .
Questions for consideration?
- How could you incorporate these virtual labs into your teaching plans?
- Are these labs better as self-paced homework assignments or small-group collaboration in class?
(1) Shaw, Vikram. (n.d.), Biochemistry Lab Techniques for the MCAT: Everything You Need to Know. Shemassian Academic Consulting. Retrieved from: https://www.shemmassianconsulting.com/blog/biochemistry-techniques-mcat#part-7-centrifugation-and-chromatography

Labster helps universities and high schools enhance student success in STEM.
Explore More

Why STEM is Fundamental to Every Student's Success and Our Collective Future
-transformed.png)
Labster's New Multi-Add Feature for LMS Courses: Streamlined Course Creation for Educators

How Labster’s Core Learning Architecture Enhances the Student Learning Experience

Ready to Increase STEM Pass Rates?
Request a demo to discover how Labster helps high schools and universities enhance student success.
Six Biochemistry Experiments for College Students
College instructors face few difficulties in finding lecture plans for teaching and doing biochemistry experiments, lesson plan sites offer an assortment of inexpensive and even free lesson plans for introducing biochemical concepts on which instructors can expand.
What is difficult for college biochemistry instructors to write are lesson plans for experiments that reinforce basic concepts in biochemistry while requiring students to articulate a hypothesis, test it in the lab, and articulate their results. Materials for teaching scientific facts are abundant, but materials for teaching scientific methods are hard to find.’
Modern Biology offers not just lesson plans but also complete lab materials for 25 experiment packages for biochemistry . Experiments from Modern Biology go beyond laboratory demonstrations.
Every experiment from Modern Biology trains students in the scientific method, not just scientific fact. And every experiment from Modern Biology includes test materials, standards, reagents, and incidental equipment for the exercise, no ordering or requisitioning is required.
Experiments for college biochemistry
Molecular Approach to study of Genetics and Evolution
Modern Biology’s O1: A Molecular Approach to the study of Genetics and Evolution helps students develop basic skills for the characterization of proteins and DNA. This module provides lesson plans and complete supplies for three experiments for 16 students for just $169.95.
Experiments in this series include;
EXP-101 Electrophoretic Separation of Proteins: This experiment introduces students to the basic technique of separating proteins by electrophoresis. They study the variation of migration rates of four proteins across and charged agarose gel. Each protein is dyed with a different color for easy identification.
EXP-102 Genetics and Sickle Cell Anemia: Students compare electrophoretic patterns in DNA from a person with normal hemoglobin with DNA from a person who has sickle cell traits. They are given an opportunity to test their own DNA for hemoglobin variants, This experiment can also serve as a springboard for discussion of social policy implications of molecular genetics.
EXP-106 Protein Fingerprinting: Students use electrophoresis to study the forms of lactate dehydrogenase found in different animals. They will note that the patterns in sheep and goat are similar to each other but very different from patterns in cow and horse.
Introduction to Molecular Genetics
Modern Biology’s Introduction to Molecular Genetics provides lesson plans and complete supplies for three experiments for 16 students for just $199.95.
EXP-301 Length of the DNA Molecule: Students determine the length of a sample of DNA by electrophoresis over an agarose gel, This experiment reinforces student understanding and practical skills for standardized ladder technique.
EXP-302 Restriction Nuclease Mapping of DNA: Bacteriophage lambda attacks E. coli DNA. In this experiment, students use restriction endonucleases EcoR1 and BamH1 to dissect bacteriophage lambda DNA to identify structures, sequences, and specific sites along the phage genome. Students gain a deeper understanding of DNA mapping strategies, complementary base-pairing of DNA, specificity of restriction endonucleases, and the structure of a viral genome.
EXP-304A Molecular Cloning: Students create a strain of E. coli that is resistant to ampicillin by introducing an ampicillin-resistance gene with a plasmid. They monitor the success of their procedure by growing E. coli in a medium including ampicillin.
How the Modern Biology experiments work
Experiments in these series are available separately. Modern Biology offers 19 other experiments on topics ranging from DNA hybridization to tissue-specific proteins.
Every experiment manufactured by Modern Biology matches a clear and concise lesson, with notes to help teachers prepare for it. Teachers can also develop test questions from the lesson guide.
All experiment kit made by Modern Biology supports scientific thinking. Students test their hypotheses. They never just watch demonstrations. They are never limited to facts and vocabulary.
Modern Biology helps students develop the manual dexterity and note-taking skills that they will carry to future study and their careers. Modern Biology is developed by working scientists for future working scientists.
Modern Biology kit includes all the reagents and test materials teachers need for their laboratory exercise. There are no ordering separate reagents, fussing about missing shipments, or checking out lab materials from the supply room.
Modern Biology supplies the safe, non-toxic, reliable reagents and measurement materials you need for every laboratory exercise . And because every Modern Biology experiment is available at a fixed cost, it’s easier to budget your supply cost for each class for each term.
Want to learn more? Email Modern Biology or call us at (765) 446-4220 Monday through Friday from 9 to 5 Eastern time.
⚠️ Due to Customs restrictions, we only accept orders from educational institutions within the Continental United States, Alaska or Hawaii. Dismiss
Search Filters:
Main Navigation
Search Cornell
Cornell Institute for Biology Teachers
- Labs & Activities
- Workshops & Events
- Equipment Lending Library
- Connect with Cornell
Labs & Activities
Browse labs & activities:.
- 2012 CIBT Alumni Workshop (19)
- Animals (18)
- Ecology (25)
- Elementary School (12)
- Evolution (10)
- Forensics (3)
- Genetics (7)
- High School (49)
- Human Health (11)
- Inquiry/Scientific Method (30)
- Insects (7)
- Microbiology (12)
- Middle School (32)
- Molecular Biology (12)
- Physical Sciences (10)
- Physiology (10)
- Plants (19)
- Recently Updated! (6)

Abuse-a-Cyst- University of Utah
2012 cibt alumni workshop, high school, inquiry/scientific method, middle school.
Brine shrimp populations survive in some of the harshest environments. Subject brine shrimp cysts to extreme conditions then try to hatch them to see just how tough they are! Downloads Abuse a Cyst Lab (University of Utah)
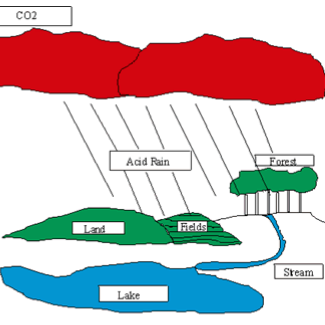
Acid Rain Lab- Katherine Betrus Derrico
Students will design and conduct an experiment to test the effect of acid rain on the germination of seeds. They will utilize the data from their experiment to explain their conclusions, and also read a passage on acid rain. Downloads Acid Rain Lab Rubric (Katherine Betrus Derrico) Acid Rain Lab… read more of the article entitled “Acid Rain Lab- Katherine Betrus Derrico”
Battle-jar Galactica- Matt Downing
Microbiology.
In this investigation students will study the types of bacteria that grow during the formation of sauerkraut, identify some characteristics of each, as well as research the type of respiratory pathway used by the organisms to break down the cabbage to get their energy. Downloads pH Questions (Matt Downing) Bacteria… read more of the article entitled “Battle-jar Galactica- Matt Downing”
Biological Shapes
Molecular biology, physical sciences.
Remember the old salad dressing commercial tag line “…because oil and vinegar don’t mix!” Water has that same love/hate relationship with many other molecules. Through this series of lessons, students will learn about the properties that make other molecules “love” or “hate” water. They will also begin to build a… read more of the article entitled “Biological Shapes”
Biomagnification Lab- Todd Shuskey
This lab demonstrates how contaminants can accumulate in organisms within a food web by using paper cutouts and M&M®s candies to simulate fish, osprey, and DDT. Students can see how the contamination levels increase as the trophic level increases. Downloads Biomagnification Lab Pictures (in color) Biomagnification Lab Pictures (in black and… read more of the article entitled “Biomagnification Lab- Todd Shuskey”
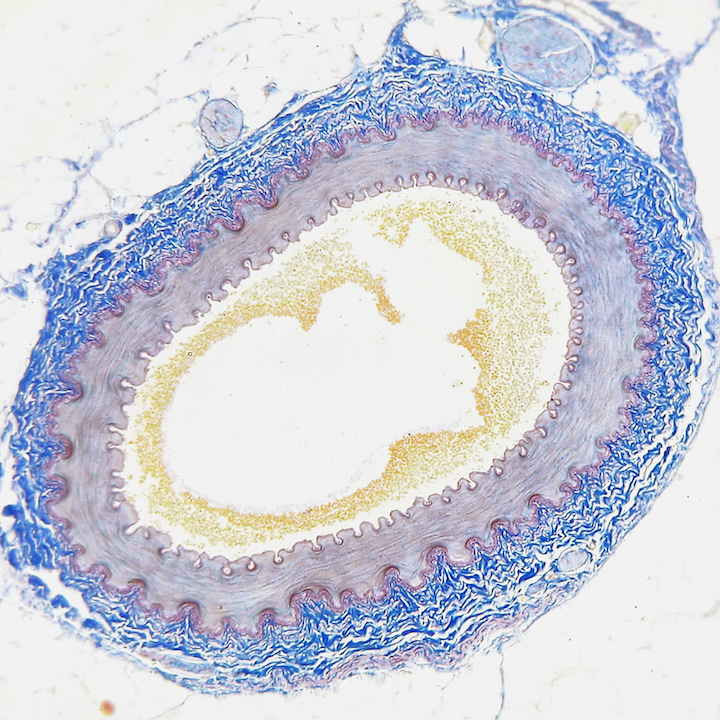
Blood Vessel Physiology
Human health.
This lab investigates the physical mechanisms by which our blood vessels function in allowing the circulatory system to do its job. Blood vessels are not simply rigid tubes that conduct blood to our tissues like copper pipes carrying water to our houses, nor are they infinitely extensible balloons that can… read more of the article entitled “Blood Vessel Physiology”

Bottle Ecosystem- Tim Downs
The objective of this lab is to put together a suitable habitat (ecosystem) that will allow one or two guppies to survive to the end of the school year and beyond. Students will make observations of their ecosystems for the three weeks. The ecosystem in this experiment will be closed,… read more of the article entitled “Bottle Ecosystem- Tim Downs”

Bouquet of Flowers
Recently updated.
This series of four different lab activities all relate to flower reproduction. They have been designed to relate to each other and to stand alone. Name that Pollinator focuses on adaptations for successful pollination. Both pollen and pollen vectors are examined. Observing, data gathering, making measurements through the microscope, and… read more of the article entitled “Bouquet of Flowers”
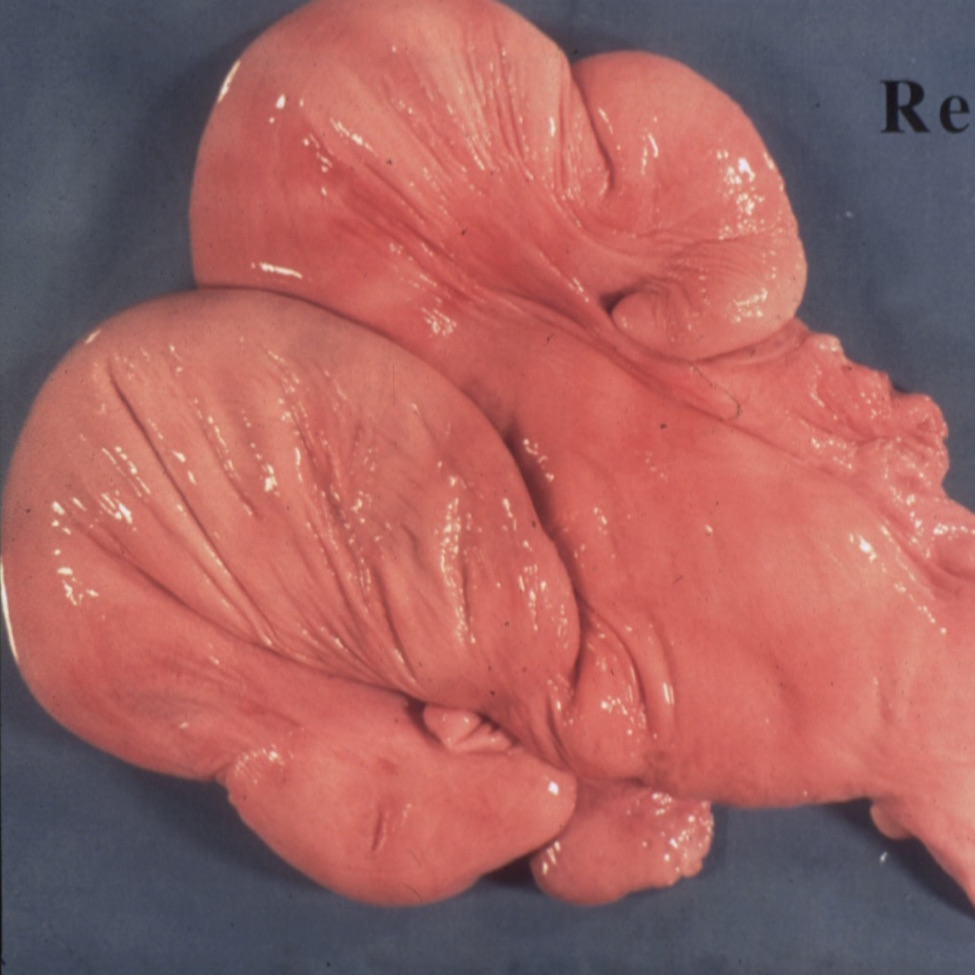
Bovine Uterus Dissection
The instructor will dissect an early to mid-pregnant bovine reproductive tract. Data on crown rump length and fetal mass can then be collected for use with CIBT’s Fetal Development Lab. Some appreciation of the form and function of the various organs should be developed by students. This exercise will also… read more of the article entitled “Bovine Uterus Dissection”
Building Blocks of Life
The shape of a protein determines its function. In this lab, students will be given a hypothetical DNA sequence for part of an enzyme. Using the Universal Genetic Code, they will then determine the amino acid sequence coded for by the DNA. Students will examine a “substrate” and predict the… read more of the article entitled “Building Blocks of Life”
The structure and function of enzymes is a central theme in cellular and molecular biology. In this laboratory exercise, a crude cell extract is prepared from potatoes. Activity of the enzyme, catalase [which catalyzes the reaction 2H2O2(l) → 2H2O(l) + O2(g)], is then studied using a simple assay for O2. To… read more of the article entitled “Catalase”

Comparative Skulls
What can a skull tell you? A lot! If you look at a skull for clues about its origin, not only can you identify what species it might be from, but you can learn many details about the original animal. In this lab, students will determine what clues to analyze in… read more of the article entitled “Comparative Skulls”
Comparing Aquatic Communities
Teams of students measure physical and chemical characteristics of different sites in streams and/or ponds and collect benthic invertebrate organisms. They interpret patterns in the structure of the biological community at each site in light of the abiotic (physical and chemical) and biotic nature of the environment. Downloads Comparing Aquatic… read more of the article entitled “Comparing Aquatic Communities”

This lab uses two different sizes of dialysis tubing to represent cellular and organelle membranes. Students design experiments in which they place solutions of iodine, starch, and glucose on different sides of a membrane. The movement of these materials is monitored with the use of indicator solutions. Students are given… read more of the article entitled “Diffusion”
DNA Molecule Activity
This lab activity corresponds to CIBT’s DNA Molecule Model. Downloads DNA Molecule HS Student Edition (CIBT) DNA Molecule MS Student Edition (CIBT) DNA Molecule Post-Lab Questions (CIBT) Watson & Crick Reading (CIBT) Watson&Crick Reading Qs Student Edition (CIBT) Watson&Crick Reading Qs Teacher Edition (CIBT) DNA Blankenship Notes (CIBT)
-->Cornell University --> ©2014 ↑

5 biology experiments you can do at home
Try you hand in biochemistry!
Safety precautions
Conduct this experiment only under adult supervision.
How can you isolate DNA in your own kitchen?
DNA is a molecule with a complex structure that stores and passes on the genetic information of every living organism on Earth. This simple method of DNA extraction is based on using surfactants (soap) to destroy the lipid layers of banana cells’ membranes and nuclei. Then, sodium ions from the table salt convert the DNA molecules from the destroyed cell material into a form that easily precipitates from the cold isopropyl alcohol.
What is kombucha? Just a drink, or …?
A SCOBY (Symbiotic Culture Of Bacteria and Yeast) is a symbiotic organism consisting of two components: bacteria and yeast, as the name suggests. The yeast ferments sugar to form alcohol and carbon dioxide, and acetic acid bacteria oxidize the alcohol and convert it into organic acids. They gradually create a complex system that forms a thin film on the liquid's surface. As the bacteria and yeast multiply, the film thickens and acquires a characteristic jellyfish-like shape. This gives it its name: Medusomyces gisevii. Be sure to wash the glass container thoroughly with a baking soda solution and rinse it with boiling water to prevent the formation of the usual mold instead of SCOBY.
How to use cabbage to make pH indicator strips Red cabbage contains pigments known as anthocyanins. Anthocyanins can also be found in many fruits, vegetables, and berries, such as blueberries, red grapes, red onions, and so on. They change colors in accordance with the acidity of their environment – a property that can help you determine the pH of various substances around you! They turn red in acidic mediums such as vinegar, purple in weakly acidic and neutral mediums such as water, blue in weakly basic mediums such as a solution of baking soda, and green, then yellow in strongly basic solutions such as drain cleaner.
How to test for vitamin C at home
Many fruits and vegetables contain ascorbic acid, also known as vitamin C . It plays a key function in biological processes in the body as a good reducer and consequently a strong antioxidant. It helps limit the impact of various free radicals (oxidizing agents that can cause mutations and destruction in cells) on the organism. Iodine can be used to test vegetables and fruits for ascorbic acid content. Iodine is an oxidizer, so when it reacts with ascorbic acid, it is reduced to colorless iodide ions.
How to obtain oxygen with the help of a plant
Here is an interesting and entertaining experiment that lies on the borderline of two sciences – chemistry and biology. You can easily try it at home and amaze your friends and family. Photosynthesis is a complex chemical process in which light energy is transformed into chemical bond energy. More simply, it is a process in which carbon dioxide and water are made into organic substances and oxygen under the influence of light.
It is easy to prove the presence of oxygen in the test tube. As oxygen is a gas that supports combustion, all you have to do is lower a smoldering splint or match into the test tube, and it will immediately flare up.
Why do we need a solution of baking soda? As the carbon dioxide in the air dissolves poorly in water, we can use carbonates or bicarbonates, which by their nature are salts of carbonic acid, to increase its concentration.


Dozens of experiments you can do at home
One of the most exciting and ambitious home-chemistry educational projects The Royal Society of Chemistry

Home Biochemistry Experiments: Discovering Enzymes, Proteins, and DNA
Unlock the secrets of biochemistry with our "Home Biochemistry Experiments" guide! This concise, one-page resource features five exciting experiments designed to be conducted at home. Students will explore enzyme activity, protein precipitation, starch detection, and more through simple, hands-on activities. Perfect for science enthusiasts, homeschooling, or classroom enrichment.
Key Features:
Five Hands-On Experiments: Engage with biochemistry concepts through practical activities like enzyme testing, protein precipitation, and DNA extraction.
Clear Instructions: Step-by-step guidance ensures easy setup and execution for each experiment.
Minimal Equipment Required: Most materials are commonly found at home or easily accessible.
Educational Outcomes: Each experiment includes learning objectives to deepen understanding of biochemistry principles.
Suitable for All Ages: Designed to be accessible for various age groups and educational levels.
How to Use It:
Gather Materials: Collect the materials listed for each experiment. All items are either common household goods or readily obtainable.
Follow Instructions: Adhere to the detailed steps for each activity, ensuring safety and proper handling of materials.
Observe and Record: Document observations, noting any changes or reactions. Take photos if possible to compare results.
Discuss Results: Review the findings together, discussing the biochemical processes observed and their implications.
Expand Learning: Utilize the educational outcomes provided to further explore and understand biochemistry concepts.
This guide offers a straightforward and engaging way to delve into biochemistry, making complex topics accessible and enjoyable through practical experimentation.
Order your copy today!
Check out our blog for more information - Click Here
You Might Also Like

Top Ten Projects
- Candle Race
- Home-Made Glue #1
- Soil Erosion
- Volcanic Gas
- Accelerate Rusting
- Vibrating Coin
- Mentos Soda Volcano
- Musical Bottles
- Human Battery Power
Latest Projects
- Sweet Erosion
- Your Planetary Age
- Exploding Ziploc
- Dehydrated Potato
- Homemade Windmill
Want to contribute?
Biochemistry.
Biochemistry is the science and study of vital processes and chemical substances occurring within living organisms, including carbohydrates, proteins, lipids, minerals, hormones, and more.
All projects
To demonstrate that carbon dioxide is necessary for plant leaves to carry out photosynthesis
To collect separate and collect DNA from a source using common household items.
To determine whether or not different detergents affect the growth and health of plants and if they do, to measure in what ways the plants have been affected.
The purpose of this experiment is to find out which type of fertilizer, whether organic or inorganic, speeds the growth of plants.
To determine whether different colors of light hinder or aid in the growth of common bean plants.
To determine whether plants grow better when exposed to classical music or whether they grow better without being exposed to classical music, or if it doesn’t make a difference.
To determine whether seeds germinate better in wet paper towels or in soil.
To determine whether a bean plant grows faster and taller using a traditional soil planting method or hydroponic method.
All Projects List
All categories.
home | about us | support | link to us | usage agreement | privacy policy | sitemap article resources -->
Copyright 2007, Sciencefairadventure.com. All Rights Reserved.
Browse Course Material
Course info.
- Dr. Elizabeth Vogel Taylor
Departments
As taught in.
- Biochemistry
Learning Resource Types
Biochemistry laboratory.
Complete student manual ( PDF )
Supplemental TA instructions ( PDF )
Table of contents ( PDF )
Appendix A-D and references ( PDF )
Equipment and supplies list ( PDF )
The table below contains links to the instructions for each lab session, as well as links to photographs of the labs.

You are leaving MIT OpenCourseWare

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Laboratory Experiments
- Last updated
- Save as PDF
- Page ID 3731
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Online lab work
Lab courses are one of the more complicated and difficult spaces to recreate or instruct through remote learning. Accept that the experience cannot be fully reproduced. Again, this is something that can be backward-designed. Start with the key learning objectives, and let that drive your decisions. If data analysis and critical thinking are part of your laboratory class outcomes, ensure they are built into the design of your lab course. AND let yourself to be creative and imperfect in design of the class.
For lower level laboratories, are there simple experiments that can be conducted at home ? Depending on the number of students, mailed pH strips and household items could be used in a titration. Alternatively, consider finding a virtual simulation from the many collections on the Internet. We all know making solutions and buffers are the bane of biochemistry students - so think about designing learning activities on these subjects.
Deep analysis of the literature is also an excellent way to build laboratory-related skills. Access a Journal of Biological Chemistry article and have the students review the methods sections - they can create presentations on various aspects of the methods of a few key papers and analyze the figures and results sections in a follow-up activity.
Demonstrations are very valid when in a pinch. You could create a video of a technique and annotate the key steps of a western blot, an SDS-PAGE gel, or protein assay, or keep your teaching assistants employed by having them create videos or demonstrations. Include simple quizzes to keep students engaged. Walk students through an enzyme assay (use some of the resource links below if you don’t have your own) and videotape the assay being conducted - perhaps even do this live in a Zoom or WebEx chat or using the meeting features in your LMS. Show the spectrophotometer as absorbance changes to talk about reactants and products. Pause and interact with the students, have them break out into groups (if your LMS allows) and make predictions on the experiment at hand. Then give them data sets to analyze. You might assign different data sets for different student groups, perhaps some that have a “flaw” and a follow-up repeated experiment.
Biomolecular modeling is another interactive option. Using Chimera, PyMOL, Jmol or other visualization programs, you can design experiments where students are asked to model active sites, mutate amino acids, and consider the effects of a proposed mutation. There are RNAseq and protein mass spectroscopy databases that can be mined for hypothesis-driven experiences. Free programs exist to modify a PDB file and predict protein or ligand docking and energy minimization. Ask students to make predictions and conduct a virtual experiment based on a PDB structure. You can take advantage of many online tutorials in this area; there are several linked examples of these in the resource list.
Shared Data Sets. If you can use data sets from your prior labs, your personal research or that of a colleague, you can set up the kinds of virtual labs discussed here. Two specific examples linked below that focus on protein biochemistry are the Malate Dehydrogenase CURE Community and BASIL virtual protein lab groups. Both have plans for running parts of a semester in virtual format using existing shareable data. CourseSource and CURE.net are other possible sources to find labs with data to convert to this format.
You could also consider having students work on a grant proposal as an alternative to wet lab techniques. Students will gain experience in formulating hypotheses, experimental design, and literature analysis. They can learn about techniques and instrumentation that they might not have available to them in the laboratory.
Finally, think of all of the times you’ve taught a laboratory and wished there was more time to have students really think about their results… Why not have them go back and be critical in their analysis? Now you have time to do some very valuable teaching to the students.
- General considerations Taken from a thread: three approaches to moving labs to an online format, resources.
Lab videos/Simulations
- LabXchange Wide collection of labs. Biotechnology/molecular biology simulators
- Merlot Wide collection of labs, some rated.
- Simulated labs by iWorx of animal physiology Data and results (.zip download)
- Interactive biology simulations (i.e. neuron, density)
- Interactive chemistry simulation
- Webmo Web-based interface to computational chemistry packages.
- Chem Collective Free chemistry lab simulations by topic and type of source.
- Chemical reaction simulator and acid base titrations Free web tool.
- Titrations (acid base and redox)
- Pearson LabBench Activities - simple lab demonstractions for introductory biology
- Learn.Genetics - Biochem/mol bio/genetics/neuroscience- virtual experiments with content explained
- Virtual labs - simple demonstrations of bio/mol bio labs
- Bio-Rad Explorer - Instructional videos that demonstrate various lab techniques
- Exploration of an interactive “Virtual and Actual Combined” teaching mode in medical developmental biology
Virtual labs
- Malate Dehydrogenase CUREs Community example of moving to a virtual lab class.
- Biochemistry Authentic Scientific Inquiry Laboratory CUREs First five modules are computational based and amenable to virtual learning.
- Bruker NMR Topspin Free NMR topspin licensing for academics.
- HHMI BioInteractive Biochemistry/Mol Bio interactive lab simulations.
- Interactive ELISA assay
- Practical Biochemistry: Everyday Applications & Examples
Biochemistry in Everyday Life: Practical Applications and Examples

Biochemistry is a fundamental branch of science that explores the chemical processes and substances within living organisms. While its complex theories and laboratory experiments are often associated with academic settings, the principles of Biochemistry permeate various aspects of our daily lives, often in subtle yet significant ways. This blog aims to illuminate the practical applications and examples of biochemistry that we encounter in our everyday routines, shedding light on its relevance beyond the confines of scientific research. Understand its real-world implications, this guide will provide valuable insights into the vital role biochemistry plays in our daily lives.
One of the most recognizable applications of biochemistry in everyday life is in nutrition. The food we consume undergoes intricate biochemical processes within our bodies to provide energy, essential nutrients, and support bodily functions. For instance, carbohydrates are broken down into glucose, which serves as a primary source of energy for cells through the process of glycolysis. Similarly, proteins are digested into amino acids, which are crucial for building and repairing tissues, while lipids provide energy storage and insulation. Understanding these biochemical processes helps individuals make informed dietary choices to support their overall health and well-being.
Another prominent example lies in the realm of medicine and healthcare. Biochemistry underpins the mechanisms of various drugs and medications used to treat diseases and alleviate symptoms. For instance, antibiotics target specific biochemical pathways in bacteria, disrupting their ability to replicate and survive. Similarly, chemotherapy drugs interfere with the biochemical processes involved in cancer cell proliferation, offering therapeutic benefits to patients. Moreover, biochemical analyses, such as blood tests and enzyme assays, play a vital role in diagnosing diseases and monitoring patient health.
Beyond nutrition and healthcare, biochemistry influences our daily interactions with the environment and consumer products. For instance, the process of fermentation, a biochemical reaction mediated by microorganisms, is harnessed in the production of bread, cheese, yogurt, and alcoholic beverages. Additionally, biofuels derived from organic matter utilize biochemical processes such as fermentation and photosynthesis to generate renewable energy sources. Furthermore, the development of biodegradable plastics and sustainable materials leverages biochemistry principles to create eco-friendly alternatives to traditional petroleum-based products.
In the realm of personal care and cosmetics, biochemistry contributes to the formulation of skincare products, cosmetics, and fragrances. Ingredients such as vitamins, antioxidants, and botanical extracts undergo biochemical interactions to provide various benefits, such as moisturization, anti-aging effects, and UV protection. Similarly, enzymes and surfactants play key roles in the cleansing and conditioning properties of shampoos and soaps, highlighting the intersection of biochemistry with beauty and grooming routines.
Biochemistry Basics for Everyday Understanding
Biochemistry is the scientific study of the chemical processes and substances that occur within living organisms. While it may seem like a complex and specialized field reserved for scientists in laboratories, understanding the basics of biochemistry can offer valuable insights into various aspects of our everyday lives. This blog aims to demystify biochemistry by exploring its fundamental concepts and illustrating their relevance in our daily experiences.
At its core, biochemistry delves into the molecular mechanisms that govern life. It explores how molecules such as proteins, carbohydrates, lipids, and nucleic acids interact and function within cells to maintain essential processes like metabolism, growth, and reproduction. Understanding these molecules and their roles provides a foundation for comprehending the biochemical processes underlying phenomena we encounter in everyday life.
One of the central concepts in biochemistry is the idea of metabolism, which encompasses all the chemical reactions that occur within a living organism to sustain life. Metabolism involves processes like energy production, molecule synthesis, and waste elimination. For instance, the breakdown of carbohydrates into glucose through glycolysis is a metabolic process that provides energy for cellular activities. Similarly, the synthesis of proteins from amino acids is another essential metabolic process that supports tissue growth and repair.
Another key concept in biochemistry is enzyme activity. Enzymes are specialized proteins that catalyze biochemical reactions, speeding up the rate at which they occur without being consumed in the process. Enzymes play crucial roles in various bodily functions, including digestion, cellular signaling, and metabolism regulation. Understanding enzyme activity helps us appreciate the efficiency and specificity of biochemical reactions, such as the digestion of food by digestive enzymes or the conversion of substrates into products in metabolic pathways.
Biochemical principles also underpin our understanding of nutrition and the impact of diet on health. The macronutrients—carbohydrates, proteins, and fats—provide energy and essential building blocks for cellular processes. By understanding how these nutrients are digested, absorbed, and utilized by the body, individuals can make informed dietary choices to support their overall well-being. For example, knowing that carbohydrates are broken down into glucose for energy can guide decisions about carbohydrate intake and blood sugar management.
Moreover, biochemistry intersects with various industries and technologies that affect our daily lives. From pharmaceuticals and healthcare to food production and environmental sustainability, biochemical principles inform advancements and innovations that shape our world. For instance, the development of biotechnologies like genetic engineering and bioremediation relies on an understanding of biochemistry to manipulate biological systems for practical purposes.
Understanding Biomolecules: The Building Blocks of Life
Biomolecules are the molecules that make up living organisms. These include carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates serve as the primary source of energy for our bodies, while proteins are essential for various functions such as growth, repair, and immune response. Lipids, including fats and oils, play a vital role in energy storage and insulation. Nucleic acids like DNA and RNA are responsible for storing and transmitting genetic information.
Understanding the structure and function of these biomolecules helps us make informed choices about our diet and lifestyle. For example, consuming a balanced diet that includes adequate amounts of carbohydrates, proteins, and fats ensures proper nutrition and overall well-being.
Enzymes: Nature's Catalysts
Enzymes are biological catalysts that facilitate biochemical reactions within living organisms. These proteins speed up chemical reactions by lowering the activation energy required for the reaction to occur. Enzymes are involved in various metabolic processes, including digestion, energy production, and DNA replication.
For example, digestive enzymes such as amylase, lipase, and protease break down carbohydrates, fats, and proteins, respectively, into smaller molecules that can be absorbed and utilized by the body. Without these enzymes, the process of digestion would be inefficient, leading to nutritional deficiencies and health issues.
Cellular Respiration: Energy Production in Action
Cellular respiration is the process by which cells break down glucose and other organic molecules to produce adenosine triphosphate (ATP), the primary energy currency of the cell. This process occurs in multiple steps, including glycolysis, the citric acid cycle, and oxidative phosphorylation.
The energy generated through cellular respiration powers various cellular activities, including muscle contraction, nerve impulse transmission, and protein synthesis. Without cellular respiration, living organisms would not be able to generate the energy required to sustain life.
The Role of DNA: Genetic Information Storage and Retrieval
DNA, or deoxyribonucleic acid, is the hereditary material found in all living organisms. It contains the instructions necessary for the development, growth, functioning, and reproduction of an organism. DNA is composed of nucleotides, which consist of a sugar molecule (deoxyribose), a phosphate group, and a nitrogenous base (adenine, thymine, cytosine, or guanine).
The discovery of the structure of DNA by James Watson and Francis Crick in 1953 revolutionized the field of genetics and molecular biology. Today, our understanding of DNA has led to advancements such as genetic engineering, gene therapy, and DNA fingerprinting, which have practical applications in various fields, including medicine, agriculture, and forensic science.
Biochemistry in Nutrition and Health
Biochemistry plays a crucial role in understanding the relationship between nutrition and health. This interdisciplinary field examines the biochemical processes involved in the metabolism of nutrients and their impact on physiological functions, disease prevention, and overall well-being. By elucidating the intricate biochemical pathways underlying nutrition, researchers and healthcare professionals can devise effective strategies for promoting optimal health and managing various health conditions.
Nutrition, at its core, revolves around the intake and utilization of essential nutrients by the body. Biochemistry provides insights into how macronutrients (carbohydrates, proteins, and lipids) and micronutrients (vitamins and minerals) are metabolized to fulfill energy requirements, support cellular functions, and maintain homeostasis. For instance, carbohydrates undergo digestion and subsequent conversion into glucose, which serves as a primary source of energy for cells through glycolysis and oxidative phosphorylation pathways. Proteins are broken down into amino acids, essential for protein synthesis, tissue repair, and enzymatic functions. Lipids are metabolized through beta-oxidation and lipogenesis pathways to produce energy and structural components for cell membranes.
Moreover, biochemistry sheds light on the biochemical mechanisms underlying the health benefits of various dietary components. For instance, antioxidants such as vitamins C and E and phytochemicals found in fruits and vegetables exert protective effects against oxidative stress and inflammation, contributing to the prevention of chronic diseases like cardiovascular disease and cancer. Similarly, dietary fiber, composed of complex carbohydrates resistant to digestion, plays a vital role in regulating blood sugar levels, promoting gastrointestinal health, and reducing the risk of obesity and type 2 diabetes.
Biochemistry also informs dietary recommendations and interventions for managing health conditions. For example, individuals with diabetes benefit from understanding the role of insulin in glucose metabolism and how dietary carbohydrate intake affects blood sugar levels. By incorporating biochemistry principles, healthcare professionals can tailor dietary plans to optimize glycemic control and reduce the risk of diabetic complications. Similarly, biochemistry elucidates the mechanisms underlying conditions like hyperlipidemia and hypertension, guiding dietary interventions aimed at improving lipid profiles and blood pressure through targeted nutrient modifications.
Furthermore, biochemistry is integral to the development of nutritional supplements and functional foods designed to address specific health concerns. Nutraceuticals, which encompass vitamins, minerals, amino acids, and herbal extracts, leverage biochemistry insights to enhance bioavailability, efficacy, and safety. By understanding the biochemical interactions between nutrients and physiological processes, researchers can formulate evidence-based nutritional interventions to support overall health and well-being.
Carbohydrates: Fuel for the Body
Carbohydrates are one of the three macronutrients essential for human nutrition, along with proteins and fats. They serve as the primary source of energy for the body, particularly for brain function and physical activity. Carbohydrates are classified into simple carbohydrates (sugars) and complex carbohydrates (starches and fibers).
Simple carbohydrates, such as glucose and fructose, are found in fruits, vegetables, and honey, while complex carbohydrates are found in grains, legumes, and starchy vegetables. Consuming a diet rich in complex carbohydrates, along with fiber, helps regulate blood sugar levels, promote satiety, and support digestive health.
Proteins: The Body's Workforce
Proteins are large, complex molecules made up of amino acids, which are essential for the structure, function, and regulation of the body's tissues and organs. They play a crucial role in various physiological processes, including muscle contraction, immune response, and hormone production.
Dietary protein sources include meat, poultry, fish, eggs, dairy products, legumes, nuts, and seeds. Consuming an adequate amount of protein is essential for maintaining muscle mass, supporting immune function, and promoting overall health and well-being.
Fats: Essential Energy Reserves
Fats, also known as lipids, are a concentrated source of energy that provides more than twice the calories per gram compared to carbohydrates and proteins. They serve as essential energy reserves, insulation for the body, and precursors for hormone production.
Dietary fats are classified into saturated fats, unsaturated fats, and trans fats. Sources of healthy fats include avocados, nuts, seeds, olive oil, and fatty fish, while unhealthy fats are found in fried foods, baked goods, and processed snacks. Consuming a balanced diet that includes healthy fats in moderation is essential for maintaining cardiovascular health and overall well-being.
Micronutrients: Small Molecules, Big Impact
Micronutrients are essential vitamins and minerals that are required in small amounts for various physiological functions in the body. They play a crucial role in energy metabolism, immune function, and overall health and well-being.
Vitamins are organic compounds that are essential for normal growth and development, while minerals are inorganic elements that are necessary for various biochemical reactions in the body. Examples of micronutrients include vitamin C, vitamin D, calcium, iron, and zinc. Consuming a diverse and balanced diet that includes a variety of fruits, vegetables, whole grains, and lean proteins ensures an adequate intake of micronutrients.
Biochemistry in Industry and Technology
Biochemistry plays a crucial role in driving innovation and advancements across various industries, ranging from pharmaceuticals and agriculture to energy and environmental sustainability. This blog aims to provide a brief overview of how biochemistry intersects with industry and technology, showcasing its diverse applications and contributions in these domains.
In the pharmaceutical industry, biochemistry serves as the backbone of drug discovery, development, and production. Researchers leverage biochemical principles to understand the molecular mechanisms of diseases and identify potential drug targets. Techniques such as recombinant DNA technology and protein engineering enable the production of therapeutic proteins and biologics used to treat various medical conditions, including cancer, diabetes, and autoimmune diseases. Moreover, biochemistry-based assays and screening methods facilitate the identification and optimization of drug candidates, accelerating the drug development process.
In the realm of agriculture and food production, biochemistry drives innovations in crop improvement, animal nutrition, and food processing. Genetic engineering techniques enable the development of genetically modified crops with enhanced nutritional value, pest resistance, and environmental sustainability. Biochemical analyses of soil and plant nutrients inform precision agriculture practices, optimizing fertilizer use and crop yields while minimizing environmental impact. Additionally, enzymes and microbial fermentation processes are utilized in food processing and preservation, improving the nutritional quality and shelf life of food products.
Biochemistry also plays a pivotal role in the energy sector, particularly in the production of biofuels and renewable energy sources. Biochemical pathways such as photosynthesis and fermentation are harnessed to convert biomass, including agricultural residues and algae, into biofuels such as ethanol and biodiesel. Moreover, enzyme-based processes are employed in the production of bio-based chemicals and renewable materials, contributing to the transition towards a more sustainable and carbon-neutral economy.
In the field of environmental biotechnology, biochemistry offers solutions for waste treatment, pollution remediation, and resource recovery. Microbial enzymes and metabolic pathways are leveraged in bioremediation processes to degrade pollutants and contaminants in soil and water, restoring ecosystems and mitigating environmental degradation. Furthermore, biochemistry-based technologies such as anaerobic digestion and composting enable the conversion of organic waste into biogas and compost, providing renewable energy and nutrient-rich soil amendments.
Biotechnology: Transforming Industries
Biotechnology involves the use of living organisms, cells, and biomolecules to develop products and technologies that improve our lives. It encompasses various fields, including agriculture, medicine, environmental science, and industrial manufacturing.
Examples of biotechnological applications include genetically modified crops with enhanced nutritional value and pest resistance, biopharmaceuticals such as insulin and vaccines produced using recombinant DNA technology, and bioremediation techniques for cleaning up environmental pollutants.
Pharmaceuticals: Healing with Molecules
Pharmaceuticals are chemical substances used for the prevention, diagnosis, and treatment of diseases. Many pharmaceutical drugs are designed based on our understanding of biochemistry and molecular biology, targeting specific biochemical pathways and molecules involved in disease processes.
For example, antibiotics target bacterial enzymes involved in cell wall synthesis or protein synthesis, while anticancer drugs inhibit enzymes involved in cell proliferation or DNA replication. Understanding the biochemical mechanisms of action of pharmaceutical drugs is essential for optimizing their efficacy and minimizing adverse effects.
Environmental Applications: Biochemistry for Sustainability
Biochemistry plays a crucial role in addressing environmental challenges and promoting sustainability. Bioremediation techniques use microorganisms and enzymes to degrade or detoxify environmental pollutants, such as petroleum hydrocarbons, pesticides, and heavy metals, into less harmful substances.
Additionally, biofuels, such as biodiesel and bioethanol, are produced from renewable biological sources, such as plants and microorganisms, through biochemical processes such as fermentation and transesterification. These biofuels offer a more sustainable alternative to fossil fuels, reducing greenhouse gas emissions and mitigating climate change.
Food Science: Enhancing Flavor, Texture, and Nutrition
Biochemistry is central to the field of food science, where it is used to understand and manipulate the chemical properties of food ingredients to enhance flavor, texture, and nutritional value. Various biochemical reactions occur during food processing, cooking, and storage, influencing the sensory characteristics and nutritional quality of food products.
For example, Maillard reaction, a chemical reaction between amino acids and reducing sugars, contributes to the browning and flavor development of baked goods and roasted meats. Fermentation is another biochemical process used in food production to enhance flavor and preserve food through the action of microorganisms such as bacteria and yeast.
Conclusion:
In conclusion, the exploration of biochemistry in everyday life reveals its pervasive influence on numerous facets of our daily routines and societal interactions. From the food we consume to the products we use and the healthcare we receive, biochemistry underpins a multitude of practical applications and examples that impact our well-being and quality of life.
Through a deeper understanding of biochemical processes, individuals can make informed decisions regarding their nutrition, health, and environmental footprint. Awareness of the biochemical basis of diseases and medications empowers individuals to take proactive steps towards disease prevention and management. Moreover, the integration of biochemistry principles in industries ranging from agriculture to personal care and renewable energy fosters innovation and sustainability, contributing to the advancement of society as a whole.
By recognizing the practical applications and examples of biochemistry in everyday life, we gain a newfound appreciation for its significance in shaping our daily experiences and improving human welfare. As we continue to uncover the intricacies of biochemical pathways and their implications, there is vast potential for further innovation and discovery in harnessing the power of biochemistry to address global challenges and enhance the quality of life for individuals worldwide. Ultimately, biochemistry serves as a bridge between scientific inquiry and tangible outcomes, demonstrating its indispensable role in shaping the world we live in.
Post a comment...
Practical biochemistry: everyday applications & examples submit your assignment, attached files.

IMAGES
COMMENTS
Use these 7 biochemistry virtual lab experiments to help teach your students and keep them interested and engaged in their biochemistry coursework.
procedures in biochemistry, including protein purification and characterization, enzyme assays and kinetics, and DNA isolation and manipulation. You will also gain some familiarity with some of the types of equipment frequently used in biochemistry.
What is difficult for college biochemistry instructors to write are lesson plans for experiments that reinforce basic concepts in biochemistry while requiring students to articulate a hypothesis, test it in the lab, and articulate their results.
The below mentioned article includes a collection of fifteen experiments on biochemistry: 1. Lipids 2. Enzymes 3. Cellulose 4. Cutin 5. Hemicellulose 6. Latex 7. Phenols 8. Lignin 9. Suberin 10. Pectin 11. Chitin 12. Tannin 13. Saponification Number 14. Iodine Number 15. Detection of Ca and P in Milk. Contents: 1.
Biochemistry lab and Biochemical. This Biochemistry laboratory seeks to introduce undergraduate students techniques used in biochemistry. A collection of eleven experiments has been presented that teach students how to detect, estimate different biomolecules with simple equipment. Each experiment set introduces a theoretical principle and the
Students design experiments in which they place solutions of iodine, starch, and glucose on different sides of a membrane. The movement of these materials is monitored with the use of indicator solutions.
Mar 19, 2021 · The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
Experiments and Labs of Interest How to Extract DNA from Anything Living [ View Experiment ] Learn the basics about DNA sequences by examining some simple differences between groups of genes.
Biochemistry and cell biology science fair projects and experiments: topics, ideas, resources, and sample projects.
Try you hand in biochemistry! Conduct this experiment only under adult supervision. How can you isolate DNA in your own kitchen? DNA is a molecule with a complex structure that stores and passes on the genetic information of every living organism on Earth.
Perhaps the greatest potential danger in your laboratory work will be from corrosive substances such as strong acids and bases that readily attack human tissues. The most vulnerable part of your body is your eyes and they must be protected.
Discover biochemistry with five fun, hands-on experiments! Perfect for students, homeschoolers, or classrooms. Learn about enzymes, proteins, and DNA with easy-to-follow instructions and minimal equipment.
Dive into the fascinating world of biotechnology with science experiments that DNA analysis, biochemical reactions, and more.
Biochemistry is the science and study of vital processes and chemical substances occurring within living organisms, including carbohydrates, proteins, lipids, minerals, hormones, and more. To collect separate and collect DNA from a source using common household items.
Prepare mutant DNA samples for sequencing. Complete crystal structure viewing exercises. This section provides information on the lab assignments of the course.
EXPERIMENTS IN BIOCHEMISTRY: A HANDS-ON APPROACH, Second Edition features a variety of hands-on, classroom tested experiments that are effective in courses meeting only once a week, giving students a broad overview of the subject matter as well as a more comprehensive set of experiments for courses that wish to delve further into each of the ...
Gives a brief overview of biochemistry experiments with proteins, enzymes, carbohydrates, lipids, nucleic acids, vitamins, metabolism, electron transport, and photosynthesis including materials, procedures, and outcomes.… Varying Iron Release from Transferrin and Lactoferrin Proteins. A Laboratory Experiment
Mar 19, 2021 · The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
Using Chimera, PyMOL, Jmol or other visualization programs, you can design experiments where students are asked to model active sites, mutate amino acids, and consider the effects of a proposed mutation.
May 25, 2024 · Explore the practical side of biochemistry with real-life examples. Discover how it shapes our daily lives. Essential reading for curious minds.