
- General Categories
- Mental Health
- IQ and Intelligence
- Bipolar Disorder


Undoing in Psychology: Mechanisms, Examples, and Implications
From the unconscious depths of the human psyche emerges a fascinating phenomenon known as undoing, a psychological defense mechanism that aims to counteract or negate the impact of previous thoughts, feelings, or actions. This intriguing concept has captivated the minds of psychologists and researchers for decades, shedding light on the complex ways our minds work to protect us from emotional distress and maintain a sense of equilibrium.
Imagine a world where our every thought and action could be erased with a simple mental flick of the wrist. While reality doesn’t quite work that way, our minds have developed ingenious methods to cope with the weight of our experiences. Undoing is one such method, a psychological sleight of hand that allows us to mentally “take back” or “undo” something we’ve done or thought.
But what exactly is undoing, and why does it matter? At its core, undoing is a defense mechanism that helps us manage anxiety, guilt, or other uncomfortable emotions by symbolically negating or reversing an unacceptable thought or action. It’s like pressing the “undo” button on your computer, but for your mind. This concept plays a crucial role in psychological theory and practice, offering insights into how we navigate the complex terrain of our inner worlds.
The Roots of Undoing: A Journey Through Time
To truly appreciate the concept of undoing, we need to take a trip down memory lane. The idea of undoing didn’t just pop up overnight; it has a rich history rooted in the early days of psychoanalysis. Our first stop on this journey is the office of none other than Sigmund Freud, the father of psychoanalysis.
Freud first introduced the concept of undoing in his 1909 paper “Notes upon a Case of Obsessional Neurosis,” where he described it as a defense mechanism used by individuals with obsessional neurosis (now known as obsessive-compulsive disorder). He observed that these patients would often perform actions to symbolically cancel out or “undo” thoughts or behaviors they found unacceptable.
As time marched on, the concept of undoing evolved and expanded beyond its original context. Psychologists and researchers began to recognize its relevance in a broader range of psychological phenomena, not just in clinical settings but in everyday life as well. This evolution highlights the dynamic nature of psychological concepts and their ability to adapt and grow as our understanding deepens.
Diving Deep: Understanding the Concept of Undoing in Psychology
Now that we’ve dipped our toes into the historical waters, let’s dive deeper into what undoing really means in the realm of psychology. At its essence, undoing is a psychological maneuver that involves an attempt to cancel out or negate a previous action or thought that causes anxiety, guilt, or discomfort.
Imagine you’ve just said something hurtful to a friend in a moment of anger. The instant the words leave your mouth, you’re filled with regret. In an attempt to “take back” what you’ve said, you might immediately apologize profusely, shower your friend with compliments, or perform kind gestures. This is undoing in action – a symbolic attempt to erase or counteract the perceived wrongdoing.
It’s important to note that undoing is more than just a simple apology or correction. It’s a deeper, often unconscious process that aims to alleviate the psychological distress caused by the original thought or action. This distinction sets it apart from more conscious coping strategies like problem-solving or seeking social support.
Undoing finds its roots in psychoanalytic theory, which posits that our behaviors and thoughts are influenced by unconscious motivations and conflicts. In this context, undoing serves as a defense mechanism – a psychological shield that protects us from overwhelming anxiety or unacceptable impulses.
But how does undoing differ from other defense mechanisms? While mechanisms like denial involve refusing to acknowledge a reality, and projection involves attributing one’s own unacceptable thoughts to others, undoing is unique in its attempt to symbolically negate or reverse a thought or action. It’s like trying to rewind time in your mind, even when you know it’s impossible in reality.
The Intricate Dance: The Psychological Process of Undoing
Now that we’ve established what undoing is, let’s break down how this fascinating process actually works. Imagine undoing as a complex dance between your conscious and unconscious mind, each step carefully choreographed to maintain your psychological equilibrium.
The process typically unfolds in several stages:
1. Trigger Event: This is the thought, feeling, or action that causes discomfort or anxiety. It could be something you’ve done, said, or even just thought about.
2. Recognition: On some level, you recognize that this event conflicts with your self-image or moral standards. This recognition might be conscious or unconscious.
3. Anxiety or Guilt: The conflict generates uncomfortable emotions, usually anxiety or guilt.
4. Undoing Response: To alleviate these uncomfortable feelings, your mind initiates the undoing response. This could involve thoughts, behaviors, or rituals aimed at symbolically negating the original event.
5. Relief: If successful, the undoing process provides a sense of relief or reduction in anxiety.
It’s crucial to understand that undoing can operate on both conscious and unconscious levels. Sometimes, we’re fully aware of our attempts to “undo” something, like when we immediately apologize for a harsh word. Other times, the process happens below the surface of our awareness, influencing our behavior in subtle ways we might not even notice.
Various triggers can set this process in motion. Common situations include moments of social faux pas, instances of perceived moral transgression, or even intrusive thoughts that conflict with our self-image. The key is that these triggers create a discrepancy between our actions or thoughts and our idealized self-concept.
Undoing in Action: Examples from Everyday Life
To truly grasp the concept of undoing, it’s helpful to see how it plays out in real-life scenarios. Undoing is not confined to the therapist’s office or psychology textbooks; it’s a phenomenon that occurs in our daily lives, often without us even realizing it.
Consider Sarah, a dedicated employee who accidentally sends a confidential email to the wrong recipient. Horrified by her mistake, she immediately sends a flurry of follow-up emails, profusely apologizing and asking the recipient to delete the original message without reading it. She then stays late at work for the next week, going above and beyond her usual duties. This excessive compensatory behavior is Sarah’s way of trying to “undo” her mistake.
Or think about Tom, who has intrusive thoughts about harming his loved ones. These thoughts terrify and disgust him, as they go against everything he believes about himself. To counteract these thoughts, Tom engages in elaborate rituals of checking on his family members and repeatedly telling them he loves them. This is Tom’s unconscious attempt to undo the perceived harm of his intrusive thoughts.
These examples illustrate how undoing can manifest in various contexts, from workplace mishaps to personal relationships. It’s important to note that cultural and social factors can influence how undoing behavior presents itself. In some cultures, for instance, the concept of “saving face” might lead to more elaborate or public displays of undoing behavior.
While undoing can sometimes serve as a helpful short-term coping mechanism, allowing us to manage immediate anxiety or guilt, it can also have negative consequences if relied upon excessively. Over-reliance on undoing can prevent us from fully processing and learning from our experiences, potentially leading to a cycle of anxiety and compensatory behavior.
The Clinical Perspective: Undoing in Psychology and Psychotherapy
In the realm of clinical psychology and psychotherapy, undoing takes on particular significance. Understanding this defense mechanism can provide valuable insights into a person’s thought patterns, emotional responses, and behavioral tendencies.
Undoing can play a role in various mental health diagnoses. For instance, in obsessive-compulsive disorder (OCD), undoing often manifests as compulsive behaviors aimed at preventing perceived catastrophes. A person with OCD might repeatedly check that they’ve locked their door, each check serving as an attempt to “undo” the possibility that they left it unlocked.
In some anxiety disorders, undoing might appear as excessive apologizing or reassurance-seeking behaviors. For individuals grappling with guilt or shame, undoing can manifest as self-punishing behaviors or excessive attempts at reparation.
Therapeutic approaches that address undoing behavior often focus on helping clients become aware of this defense mechanism and its underlying causes. Cognitive-behavioral therapy (CBT), for example, might help a client identify the thought patterns that trigger undoing behaviors and develop more adaptive coping strategies.
Psychodynamic approaches, on the other hand, might explore the unconscious conflicts or past experiences that contribute to a person’s reliance on undoing. By bringing these underlying issues to light, therapy can help individuals develop more flexible and effective ways of managing anxiety and guilt.
One of the challenges in treating undoing-related issues is that the behavior often provides short-term relief from anxiety or guilt. This immediate reinforcement can make it difficult for individuals to give up their undoing behaviors, even when they recognize that these behaviors are ultimately unhelpful.
However, understanding and working with undoing in therapy can also offer significant benefits. By helping clients recognize their undoing behaviors and the emotions driving them, therapists can guide individuals towards more authentic self-expression and healthier ways of coping with difficult thoughts and feelings.
Pushing Boundaries: Recent Research and Future Directions
As our understanding of the human mind continues to evolve, so too does our knowledge of undoing. Recent research has shed new light on this fascinating psychological phenomenon, opening up exciting avenues for future exploration.
One area of current research focuses on the neurobiological underpinnings of undoing. Using advanced brain imaging techniques, researchers are beginning to map out the neural circuits involved in this defense mechanism. Early findings suggest that undoing may involve complex interactions between regions associated with emotion regulation, cognitive control, and self-referential processing.
Another intriguing line of inquiry examines the potential adaptive functions of undoing. While traditionally viewed primarily as a defense mechanism, some researchers argue that undoing may serve important social and emotional regulation functions. For instance, undoing behaviors might help maintain social harmony by providing a means of symbolic reparation for perceived transgressions.
Emerging theories are also exploring the role of undoing in the context of forgiveness and reconciliation . Some researchers propose that undoing might be a crucial step in the process of forgiving oneself or others, providing a psychological bridge between the acknowledgment of wrongdoing and the act of moving forward.
The concept of undoing is finding applications beyond the realm of clinical psychology as well. In the field of behavioral economics, for example, researchers are exploring how undoing-like behaviors might influence decision-making and risk assessment. In organizational psychology, understanding undoing could provide insights into workplace dynamics and conflict resolution strategies.
As for future research, there’s still much to explore. Some potential areas for investigation include:
1. The development of undoing behaviors across the lifespan 2. Cultural variations in undoing and their implications 3. The relationship between undoing and other psychological constructs like reversibility and redirection 4. The potential role of undoing in resilience and post-traumatic growth
These exciting directions promise to deepen our understanding of undoing and its role in human psychology, potentially leading to new therapeutic approaches and insights into the complexities of the human mind.
Wrapping Up: The Undeniable Importance of Undoing
As we come to the end of our exploration into the fascinating world of undoing in psychology, it’s clear that this concept is far more than just an academic curiosity. From its roots in early psychoanalytic theory to its relevance in modern clinical practice and cutting-edge research, undoing continues to offer valuable insights into the workings of the human mind.
We’ve seen how undoing serves as a psychological defense mechanism, helping us manage anxiety, guilt, and other uncomfortable emotions by symbolically negating or reversing unacceptable thoughts or actions. We’ve explored its process, from the trigger event to the relief it can provide, and examined how it manifests in everyday life through relatable examples.
The clinical significance of undoing cannot be overstated. For mental health professionals, understanding this mechanism can provide crucial insights into a client’s thought patterns and behaviors, informing more effective therapeutic approaches. For individuals, recognizing undoing in their own lives can be a step towards more adaptive coping strategies and improved emotional well-being.
But the importance of undoing extends beyond the therapist’s office. In our daily lives, being aware of undoing can help us better understand our own reactions and those of others. It can provide a compassionate lens through which to view seemingly irrational behaviors, reminding us of the complex emotional landscapes we all navigate.
As research in this area continues to evolve, we can look forward to even deeper insights into the nature of undoing and its role in human psychology. From neurobiology to social psychology, the study of undoing promises to shed light on fundamental aspects of how we think, feel, and interact with the world around us.
In conclusion, undoing stands as a testament to the incredible complexity and adaptability of the human mind. It reminds us that even our most puzzling behaviors often serve a purpose, reflecting our ongoing efforts to maintain psychological equilibrium in a complex and often challenging world. By continuing to explore and understand concepts like undoing, we not only advance the field of psychology but also gain valuable tools for navigating our own inner worlds.
So the next time you find yourself frantically trying to “take back” a regrettable action or thought, remember: you’re not just fumbling or overreacting. You’re engaging in a sophisticated psychological process that has fascinated minds for over a century. And in that realization lies the potential for greater self-understanding and growth.
References:
1. Freud, S. (1909). Notes upon a case of obsessional neurosis. Standard Edition, 10, 155-318.
2. Baumeister, R. F., Dale, K., & Sommer, K. L. (1998). Freudian defense mechanisms and empirical findings in modern social psychology: Reaction formation, projection, displacement, undoing, isolation, sublimation, and denial. Journal of Personality, 66(6), 1081-1124.
3. Cramer, P. (2015). Understanding defense mechanisms. Psychodynamic Psychiatry, 43(4), 523-552.
4. Vaillant, G. E. (1992). Ego mechanisms of defense: A guide for clinicians and researchers. American Psychiatric Pub.
5. Blackman, J. S. (2004). 101 defenses: How the mind shields itself. Brunner-Routledge.
6. McWilliams, N. (2011). Psychoanalytic diagnosis: Understanding personality structure in the clinical process. Guilford Press.
7. Gross, J. J. (2015). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26(1), 1-26.
8. Schacter, D. L., Gilbert, D. T., & Wegner, D. M. (2011). Psychology (2nd ed.). Worth Publishers.
9. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing.
10. Beck, A. T., & Dozois, D. J. (2011). Cognitive therapy: Current status and future directions. Annual Review of Medicine, 62, 397-409.
Was this article helpful?
Would you like to add any comments (optional), leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Post Comment
Related Resources

Pioneers of Psychology: Trailblazers Who Shaped Modern Mental Science

Tricky Psychological Questions That Challenge Your Mind

Unconditional Love Psychology: Exploring the Science Behind Boundless Affection

Tunnel Vision Psychology: Exploring Its Impact on Perception and Behavior

Memory Tests in Psychology: Exploring Various Types and Their Applications

Sensory Neurons: Definition, Function, and Importance in Psychology

Triangulation Psychology: Unraveling Complex Relationship Dynamics

Types of Attitude in Psychology: Exploring the Spectrum of Human…

Emblems in Psychology: Exploring Symbolic Representations and Their Impact on…

Psychological Assessment Types: A Comprehensive Guide to Mental Health Evaluations
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The Undoing Effect of Positive Emotions
Barbara l fredrickson, roberta a mancuso, christine branigan, michele m tugade.
- Author information
- Copyright and License information
Address all correspondence to Department of Psychology, University of Michigan, 525 East University Avenue, Ann Arbor, Michigan 48109-1109; [email protected]
Positive emotions are hypothesized to undo the cardiovascular aftereffects of negative emotions. Study 1 tests this undoing effect. Participants ( n = 170) experiencing anxiety-induced cardiovascular reactivity viewed a film that elicited (a) contentment, (b) amusement, (c) neutrality, or (d) sadness. Contentment-eliciting and amusing films produced faster cardiovascular recovery than neutral or sad films did. Participants in Study 2 ( n = 185) viewed these same films following a neutral state. Results disconfirm the alternative explanation that the undoing effect reflects a simple replacement process. Findings are contextualized by Fredrickson’s broaden-and-build theory of positive emotions ( B. L. Fredrickson, 1998 ).
Positive emotions feel good. Plus, the balance of people’s positive and negative emotions contributes to their judgments of life satisfaction ( Diener & Larsen, 1993 ). Beyond this, however, positive emotions may also be useful, pointing to reasons for the pursuit of happiness beyond intrinsic enjoyment. Existing evidence suggests that positive emotions reliably alter people’s thinking and actions. Together with this past work, the experiments described in this article suggest that one reason positive emotions are worth pursuing is that they can help regulate negative emotions.
EFFECTS OF NEGATIVE EMOTIONS
Negative emotions can be viewed as evolved adaptations that aided our ancestors’ survival in life-threatening situations. The adaptive value of negative emotions appears to be carried by their ability to spark specific action tendencies ( Frijda, 1986 ; Lazarus, 1991 ). Anger, for instance, creates the urge to attack, fear the urge to escape, and so on. These action tendencies infuse both mind and body: As thoughts about actions narrow to these specific urges, the body mobilizes optimal physiological support for the action called forth ( Levenson, 1994 ).
Those negative emotions that create urges for specific actions requiring substantial physical energy (e.g., attack, flee) also produce heightened cardiovascular reactivity that redistributes blood flow to relevant skeletal muscles. Such cardiovascular reactivity—if large, recurrent, or prolonged—is thought to place individuals at risk for developing or exacerbating coronary heart disease ( Blascovich & Katkin, 1993 ; Williams, Barefoot, & Shekelle, 1985 ). For instance, individuals in demographic groups at greatest risk for coronary heart disease (e.g., men, African Americans, those with hostile personalities) show heightened and prolonged cardiovascular reactivity to negative emotions relative to those at lesser risk ( Anderson, McNeilly, & Myers, 1993 ; Fredrickson et al., 2000 ; Matthews & Stoney, 1988 ; Schuler & O’Brien, 1997 ; Suarez & Williams, 1989 ). Moreover, recurrent emotion-related cardiovascular reactivity appears to injure inner arterial walls, initiate atherosclerosis, and impair vascular responsiveness ( Kaplan, Manuck, Williams, & Strawn, 1993 ).
To the extent that negative emotions generate cardiovascular reactivity that may damage people’s health, it becomes critical to discover effective ways to regulate negative emotions. Certainly, effective negative emotion regulation has multiple benefits beyond health promotion, including (but not limited to) enhanced subjective well-being ( Diener & Larsen, 1993 ) and improved cognitive and social functioning ( Eisenberg, Fabes, & Losoya, 1997 ; Salovey, Bedell, Detweiler, & Mayer, 2000 ). Positive emotions may hold a key to these various benefits.
EFFECTS OF POSITIVE EMOTIONS
Although most emotion theorists who discuss specific action tendencies extend their theories to include positive emotions like joy, contentment, interest, and love (e.g., Frijda, 1986 ; Lazarus, 1991 ), Fredrickson (1998 , 2000 , in press-a ; Fredrickson & Levenson, 1998 ) has argued that such extension is unwarranted. This conclusion is based on multiple observations within the scattered empirical and theoretical literature on positive emotions. One is that the action tendencies identified for positive emotions (e.g., free activation, approach) are better described as nonspecific than specific. A second is that positive emotions are often characterized by a relative lack of autonomic reactivity ( Levenson, Ekman, & Friesen, 1990 ). To the extent that autonomic reactivity supports specific action tendencies, these two observations go hand-in-hand: If no specific action is called forth during positive emotional states, then no particular pattern of autonomic reactivity should be expected.
The psychophysiological effects and adaptive functions of many positive and negative emotions, then, do not seem isomorphic. Instead, Fredrickson (1998) proposed they are distinct and complementary: Many negative emotions narrow individuals’ thought–action repertoires by calling forth specific action tendencies (e.g., attack, flee), whereas many positive emotions broaden individuals’ thought–action repertoires, prompting them to pursue a wider range of thoughts and actions than is typical (e.g., play, explore). Experiments on the cognitive and behavioral effects of positive emotions support this claim. Most notably, Isen and colleagues have demonstrated that people experiencing positive emotions show patterns of thought that are significantly broadened and diverse ( Isen, Johnson, Mertz, & Robinson, 1985 ), flexible ( Isen & Daubman, 1984 ), creative ( Isen, Daubman, & Nowicki, 1987 ), integrative ( Isen, Rosenzweig, & Young, 1991 ), open to information ( Estrada, Isen, & Young, 1997 ), and efficient ( Isen & Means, 1983 ; Isen et al., 1991 ). People experiencing positive emotions also show increased preference for variety and broader arrays of behavioral interests ( Cunningham, 1988 ; Kahn & Isen, 1993 ). In general terms, Isen has suggested that positive emotions produce a “broad, flexible cognitive organization, and ability to integrate diverse material” ( Isen, 1990 , p. 89). She has recently linked these effects of positive emotions to increases in brain dopamine levels ( Ashby, Isen, & Turken, 1999 ).
The broadened thought–action repertoires accompanying positive emotions are thought to be important because they can build a variety of enduring personal resources ( Fredrickson, 1998 , in press-a ). Being playful, for instance, can build physical resources ( Boulton & Smith, 1992 ), social resources ( Aron, Norman, Aron, McKenna, & Heyman, 2000 ; Lee, 1983 ), and intellectual resources ( Leslie, 1987 ; Panksepp, 1998 ). The observation that positive emotions lead to increments in enduring resources suggests that the evolved adaptive value of positive emotions is distinct from that associated with negative emotions: Negative emotions are thought to promote survival in the moment of threat by sparking specific, life-preserving actions, whereas positive emotions may promote survival over the long run by incrementing the resources that could be drawn on when facing later, inevitable threats ( Fredrickson, 1998 ). Readers interested in the broaden-and-build theory of positive emotions are directed to Fredrickson (1998 , in press-a , in press-b ) for a review of the theory’s rationale and empirical support.
POSITIVE EMOTIONS AND NEGATIVE EMOTION REGULATION
The complementarity between negative and positive emotions outlined in the broaden-and-build theory has implications for negative emotion regulation. If positive emotions broaden individuals’ thought–action repertoires, they should also serve as particularly efficient antidotes for the lingering effects of negative emotions, which narrow individuals’ thought–action repertoires. In other words, positive emotions might “correct” or “undo” the aftereffects of negative emotions; we call this the undoing hypothesis ( Fredrickson & Levenson, 1998 ; Levenson, 1988 ). Earlier research on motivation ( Solomon, 1980 ), anxiety disorders ( Wolpe, 1958 ), and aggression ( Baron, 1976 ) demonstrated key incompatibilities between negative and positive affect. More recent research on self-regulation ( Aspinwall, 1998 ; Reed & Aspinwall, 1998 ; Trope & Pomerantz, 1998 ) and coping ( Folkman, 1997 ; Folkman & Moskowitz, 2000 ) has similarly shown that positive emotion may function as a resource, as individuals manage threats and stress. The present research reframes these findings in terms of the broaden-and-build theory, and posits that people might harness the effects of a range of positive emotions to regulate a range of negative emotions.
Notably, the undoing hypothesis suggests a novel relationship between positive emotions and cardiovascular reactivity. Perhaps positive emotions do not themselves generate cardiovascular reactivity, but instead quell any existing cardiovascular reactivity caused by negative emotions. Put differently, a prior state of negative emotional arousal may be a necessary backdrop to illuminate the cardiovascular impact of positive emotions ( Levenson, 1988 ). Assuming (as most emotion theorists do) that the cardiovascular reactivity sparked by certain negative emotions prepares the body for specific actions, the broaden-and-build theory suggests that positive emotions speed recovery from—or undo—this cardiovascular reactivity and return the body to mid-range levels of activation more suitable for pursuing a range of behavioral options. According to this view, positive emotions have a unique ability to down-regulate lingering negative emotions and the psychological and physiological preparation for specific action that they generate. If true, this would be one good reason to pursue positive emotions.
PRELIMINARY EVIDENCE
Fredrickson and Levenson (1998) conducted an initial test of the undoing hypothesis. They first induced cardiovascular reactivity by showing research participants a film clip that elicited fear. Immediately following this fear clip, participants were shown a second clip that varied across experimental condition to elicit (a) contentment, (b) amusement, (c) neutrality, or (d) sadness. These four films elicited comparable amounts of interest in viewers, and so none was more distracting than the others. Sadness was chosen as the negative emotion comparison because it, like the positive emotions, has not been clearly linked to a high-energy action tendency, and as such could be a contender for aiding cardiovascular recovery. Results supported the undoing hypothesis: Those who viewed either of the two positive emotion clips showed the fastest cardiovascular recovery.
OVERVIEW OF STUDIES
Although this initial experiment provided compelling preliminary support for the undoing hypothesis, a number of shortcomings and a viable alternative explanation motivated the current work. One shortcoming of the Fredrickson–Levenson experiment was that the pattern of cardiovascular reactivity elicited by the fear clip included heart rate deceleration, and sowas not the typical sympathetic response associated with fear, anxiety, and other health-damaging negative emotions. Additionally, the sample tested, though ethnically diverse, included only women. Study 1 improves upon the Fredrickson–Levenson experiment in three ways: First, the initial negative emotion (from which speed of cardiovascular recovery is assessed) was elicited by having participants prepare to deliver a speech under considerable time pressure, a more active and self-relevant aversive task than film viewing. Our expectation was that the speech task would produce negative affect plus a pattern of cardiovascular reactivity clearly indicative of increased sympathetic arousal. Second, we collected a wider array of cardiovascular measures that included continuous measures of systolic and diastolic blood pressure. Third, we tested two independent samples, each with comparable numbers of women and men, and one with African Americans oversampled. Men and African Americans are important to study because these groups are known to be at greater risk for cardiovascular disease and have exhibited greater cardiovascular reactivity to negative emotions. Learning whether positive emotions also undo negative emotions for men and African Americans would suggest practical health-promoting interventions for these at-risk populations. We hypothesized that positive emotions would be unique in their ability to speed recovery from the cardiovascular reactivity that lingers following a negative emotion. In other words, we expected to solidify empirical support for the undoing effect using stronger, more definitive tests. We also expected that the undoing effect would generalize across sexes and ethnicities.
Study 2 tests an alternative explanation for the undoing effect that contrasts undoing with replacement. The favored, undoing explanation suggests that positive emotions have a unique ability to speed recovery from negative emotions, and that this effect of positive emotions only surfaces within the context of negative emotional arousal. The alternative, replacement explanation suggests that positive emotions simply replace the cardiovascular signature of negative emotions with their own signature, which is one of low arousal. Which view holds? As Fredrickson and Levenson (1998) discussed following their initial test of the undoing effect, the distinction between undoing and replacement is potentially murky. Even so, insight can be gained by examining the cardiovascular signatures associated with the four films used in this experimental paradigm when viewed following a resting baseline. A replacement explanation would be viable if the two positive emotion films created lower cardiovascular activation than both the neutral and sad films. It would not be viable if, as we suspect, the cardiovascular reactions produced by viewing the two positive emotion films are minimal and not appreciably different from those produced by viewing the emotionally neutral film. We conducted Study 2 to test the viability of the replacement explanation. Ruling out replacement would favor the undoing explanation: that the cardiovascular effects of positive emotions emerge only when features of negative emotions are present to be undone.
Participants
Two samples of university students were tested. Each provides an independent test of the undoing hypothesis. Sample 1 included 95 university students (50% women) recruited for a study on emotions through flyers and newspaper advertisements. Each was paid $30 to participate in a series of studies lasting 2 hr. Of them, 71 were European American (50% women) and 24 were African American (50% women). Sample 2 included 75 university students (45% women) enrolled in an introductory psychology course. Each received course credit in exchange for their participation. In this sample, 58 were European American, 13 were ethnic minorities (8 Asian, 3 African American, and 2 Hispanic), and 4 were of other or unspecified ethnic backgrounds.
Subjective experiences were assessed using Emotion Report Forms (adapted from Ekman, Friesen, & Ancoli, 1980 ). Participants rated the greatest amount felt of the following emotions: amusement, anger, anxiety, contentment, disgust, fear, sadness, and surprise. Ratings were made on 9-point Likert scales (0 = none ; 8 = a great deal ).
Four videotaped film clips, each 100-s long and without sound, served as the experimental manipulation in this research. These film clips were virtually identical to those used in the initial test of the undoing effect ( Fredrickson & Levenson, 1998 ). Two clips elicited two distinct positive emotions: “Waves” shows ocean waves breaking on a beach and primarily elicits contentment (this was a different, more effective clip of waves than that used in Fredrickson & Levenson, 1998 ); “Puppy” shows a small dog playing with a flower and primarily elicits amusement. Two additional clips were used as neutral and emotion control conditions, respectively: “Sticks” shows an abstract dynamic display of colored sticks piling up and elicits virtually no emotion; “Cry” shows a young boy crying as he watches his father die and primarily elicits sadness. Emotion ratings gathered from pilot participants who viewed these four film clips are reported in Fredrickson and Levenson (1998 , Fig. 1 ). These data also confirmed that the four clips elicit comparable levels of interest.

Mean duration of cardiovascular reactivity by Film Group in Sample 1 of Study 1. Error bars represent standard errors of the means.
Cardiovascular Measures
Continuous recordings were made of six cardiovascular measures at a sampling rate of 1000 Hz. From these recordings, second-by-second averages were computed. (1) Heart rate (HR): disposable snap electrodes were placed in a bipolar configuration on opposite sides of the chest to measure the participant’s echocardiogram (ECG). (2) Finger pulse amplitude (FPA): a photoplethysmograph was attached to the distal phalange of the first finger of the nondominant hand and the trough-to-peak amplitude of each finger pulse was measured to assess the amount of blood in the tip of the finger and provide an index of peripheral vasoconstriction. (3) Pulse transmission times to the finger (PTF): the interval was timed between the R-wave of the ECG and the upstroke of the pulse wave at the finger. (4) Pulse transmission time to the ear (PTE): a photoplethysmograph was attached to the right ear and the interval was timed between the R-wave of the ECG and the upstroke of the pulse wave at the ear. The two pulse transmission times index the contractile force of the heart along with distensibility of the blood vessels ( Newlin & Levenson, 1979 ). Finally, an Ohmeda Finapres Blood Pressure Monitor (Model 2300) was used to assess beat-by-beat measures of (5) diastolic blood pressure (DBP) and (6) systolic blood pressure (SBP): a self-regulating finger cuff was attached to the middle phalange of the second finger of the participant’s non-dominant hand; a sling was used to immobilize the participant’s arm at heart level.
This set of measures was selected to allow for continuous, noninvasive assessment of cardiovascular reactivity. Although HR, and to a lesser extent DBP, are under both sympathetic and parasympathetic control, four measures (FPA, PTF, PTE, and SBP) track changes mediated solely by the sympathetic nervous system. Together, these six cardiovascular measures provide a larger window onto sympathetic activation of the cardiovascular system than does any single cardiovascular index.
Anxiety Induction
The initial negative emotion was elicited using a speech preparation task. Before the experimental session began, the experimenter told participants that they would be given precisely 60 s to prepare a 3-min speech on a to-be-determined topic. They were also told that there was a 50% chance that “the computer” would select them to deliver their speech, and that if so, a 3-min timer would appear on the video monitor, cueing them to look into the video camera and begin their speech, speaking clearly. They were told that their videotaped speech would later be shown to and evaluated by students in another study. If “by chance” they were not selected to deliver their speech, they were told that a film clip would begin on the video monitor. In actuality, no participants delivered a speech, and each viewed a film clip. This cover story was used both to boost the anxiety induced by the speech task and to justify the switch to an unrelated film clip.
Participants were tested individually by a female experimenter. (For Sample 1, participant and experimenter were matched on ethnicity.) Upon arrival, participants were seated in a comfortable chair in a small well-lit room. They were told that the study was about people’s emotional reactions, that they would be videotaped, and that their bodily reactions would be monitored using physiological sensors. After participants signed a consent form, the experimenter attached physiological sensors as described here.
The experimental session began with a 5-min adaptation period. Participants then received the videotaped instructions to “relax, and empty your mind of all thoughts, feelings and memories.” This commenced a 2-min resting baseline period, the second minute of which was used as the pretask baseline. Next, participants received videotaped instructions to begin preparing a speech on “Why you are a good friend” and were given 60 s to do so. This speech preparation task was followed by the Waves, Puppy, Sticks, or Cry film clip, which was randomly assigned, blocked by participant sex, and (in Sample 1) participant ethnicity. The film was followed by a 3-min postfilm period during which the video monitor was blank. Afterwards, participants completed one Emotion Report Form to describe how they felt while preparing their speech, and a second Emotion Report Form to describe how they felt viewing the randomly assigned film clip.
Data from Fredrickson and Levenson (1998) and Sample 1 in the present study each clearly demonstrated that the Cry film produces prolonged cardiovascular recovery relative to positive films. With this difference well established, the sadness condition was omitted when testing Sample 2. Another comparison condition to consider is a “no-film” control, in which the presumably natural course of cardiovascular recovery might be assessed and compared to that produced by positive emotions. A no-film condition is problematic for two, related, reasons. First, cardiovascular activity is sensitive to perceptual and attentional processes, as well as to emotional processes. As such, comparing responses to film viewing to “doing nothing” confounds emotional content with differences in basic cognitive demands. The neutral Sticks film provides a superior comparison condition because it holds cognitive demands constant, yet is devoid of emotion. Second, people rarely (if ever) do nothing, perhaps especially when they are experiencing a potent emotion. As such, a no-film condition is more likely to reveal heterogeneity in self-chosen emotion regulation strategies than the so-called “natural” course of emotion recovery. For these reasons, we did not include a no-film condition. (For further discussion of this issue, see Fredrickson & Levenson, 1998 .)
Overview of Analytic Strategy
We first confirmed that the speech preparation task successfully induced anxiety and cardiovascular reactivity, then conducted a manipulation check to confirm that the film clips altered subjective experience as intended. We then used a priori contrasts to test the undoing hypothesis, which is both theory-driven and directional. Specifically, we tested whether the durations of cardiovascular reactivity for participants who viewed each of the two positive films were shorter than (a) the duration of cardiovascular reactivity for those who viewed the neutral film, and (b) the duration of cardiovascular reactivity for those who viewed the sad film (Sample 1 only). We tested for sex and ethnic differences throughout. Sample 1 permitted comparisons of African Americans ( n = 24) to European Americans ( n = 71); Sample 2 permitted comparisons of various ethnic minorities ( n = 17) to European Americans ( n = 58).
Responses to the Speech Preparation Task
Subjective experience.
Analyses of the Emotion Report Forms completed for the speech preparation task confirmed that this task elicited significantly higher levels of anxiety than any other emotion. For Sample 1, mean anxiety ratings were 4.79 ( SD = 2.16). These anxiety ratings did not differ by sex. However, they did differ across ethnic groups, with African Americans reporting less anxiety ( M = 3.92, SD = 2.32) than European Americans, M = 5.08, SD = 2.04; F (1, 91) = 5.38, p < .05. Even so, anxiety received the highest mean ratings within each ethnic group. For Sample 2, mean anxiety ratings were 4.96 ( SD = 2.10), and did not differ by sex or ethnicity.
Cardiovascular Reactivity
For each participant, and for each cardiovascular measure (HR, FPA, PTF, PTE, DBP, and SBP), we determined the mean reactivity over the 60-s pretask baseline and over the 60-s speech preparation task. These means, averaged across participants are shown in Table I . 4 Within-subject t -tests confirmed that the cardiovascular reactivity during the speech task was significantly different than baseline levels for all six cardiovascular variables across both samples (see Table I ). HR increased an average of 9.80 beats/min in Sample 1 and 12.18 beats/min in Sample 2; FPA decreased an average of 0.34 mv in Sample 1 and 0.57 mv in Sample 2; PTF and PTE decreased an average 0.013 and 0.008 s, respectively, in Sample 1, and 0.008 and 0.007, respectively, in Sample 2; and DBP and SBP increased an average of 1.97 and 8.99 mmHg, respectively, in Sample 1, and 4.65 and 19.06 mmHg, respectively, in Sample 2. Cardiovascular reactivity to the speech task did not differ by sex, ethnicity, or Film Group in either sample. Taken together, these cardiovascular changes include heart rate acceleration and a clear pattern of sympathetic activation (e.g., increased blood pressure and peripheral vasoconstriction).
Mean and Standard Deviation of Cardiovascular Activity During Baseline and Speech Preparation in Study 1
Note . HR: heart rate in beats per min; FPA: finger pulse amplitude in mv; PTF: pulse transmission time to the finger in seconds; PTE: pulse transmission time to the ear in seconds; DBP: diastolic blood pressure in mmHg; SBP: systolic blood pressure in mmHg. Values represent M ( SD ).
p < .001.
Manipulation Check
Analyses of the Emotion Report Forms completed for the film clips confirmed that each altered subjective experience as expected, thus replicating data reported in Fredrickson and Levenson (1998) . Tukey pairwise comparisons revealed that Waves elicited significantly more contentment (Sample 1: M = 4.75, SD = 1.94; Sample 2: M = 4.83, SD = 1.43) than any other clip, Puppy elicited more amusement (Sample 1: M = 4.67, SD = 1.97; Sample 2: M = 5.21, SD = 1.18), and Cry elicited more sadness (Sample 1 only: M = 4.09, SD = 2.52). Modal emotion reports for Sticks were zero for all eight emotion terms, confirming its emotional neutrality. Emotion ratings did not differ by sex or ethnicity in either sample.
Duration of Cardiovascular Reactivity
The duration of cardiovascular reactivity was quantified as the time elapsed (in seconds) from the start of the film clip until each participant’s cardiovascular responses on each measure returned to within the interval defined by his or her own baseline mean on that measure plus and minus one standard deviation of that mean, and remained within this interval for 5 of 6 consecutive seconds. (Fewer than 5% of duration scores were considered missing because a measure did not return to baseline during the data collection period.) Initial analyses of duration scores for individual cardiovascular measures revealed supportive trends across all measures, but no significant effects. To increase power, we created an aggregate measure of the duration of cardiovascular reactivity by computing each participant’s mean duration score across the six cardiovascular indices, a strategy also used in Fredrickson and Levenson (1998) .
In Sample 1, across all participants, the mean duration of cardiovascular reactivity was 30.08 s ( SD = 25.80). We analyzed these data for group differences using a 4 × 2 × 2 ANOVA (Film Group × Sex × Ethnicity). No main effects for, or interactions with, Sex or Ethnicity were significant. The main effect for Film Group was the sole significant effect, F (3, 81) = 3.40, p < .05. Figure 1 presents mean durations of cardiovascular reactivity for each of the four Film Groups. The pattern of means shows that contentment and amusement clips produced the fastest cardiovascular recovery. As is typical with time-based data, the duration scores exhibited a large positive skew. To test whether the significant effect for Film Group (depicted in Fig. 1 ) was unduly influenced by outliers, we also computed a Kruskal–Wallis nonparametric test on ranked data. This more conservative test confirmed that the group differences were significant, Χ 2 (3) = 9.89, p < .05. Moreover, a priori contrasts confirmed that those who viewed the contentment clip exhibited faster recovery than those who viewed the neutral clip, t (91) = 1.79, p = .038, and faster recovery than those who viewed the sad clip, t (91) = 2.68, p = .004. Likewise, those who viewed the amusement clip exhibited faster recovery than those who viewed the neutral clip, t (91) = 1.66, p = .050, and faster recovery than those who viewed the sad clip, t (91) = 2.55, p = .006.
In Sample 2, across all participants, the mean duration of cardiovascular reactivity was 37.78 s ( SD = 35.88). Again, analyzing the duration data with a 3 × 2 × 2 ANOVA (Film Group × Sex × Ethnicity), no main effects for, or interactions with, Sex or Ethnicity emerged. As for Sample 1, the sole significant effect was that for Film Group, F (2, 63) = 3.29, p < .05. Figure 2 presents mean durations of cardiovascular reactivity for each of the three Film Groups. Again, the pattern of means shows that contentment and amusement clips produced the fastest cardiovascular recovery. As for Sample 1, a Kruskal–Wallis nonparametric test confirmed that the group differences evident in Fig. 2 remained significant when the effects of outliers were controlled, Χ 2 (2) = 5.88, p = .05. Again as for Sample 1, a priori contrasts confirmed that those who viewed the contentment clip exhibited faster recovery than those who viewed the neutral clip, t (72) = 2.43, p = .008. Likewise, those who viewed the amusement clip exhibited faster recovery than those who viewed the neutral clip, t (72) = 1.78, p = .039.

Mean duration of cardiovascular reactivity by Film Group in Sample 2 of Study 1. Error bars represent standard errors of the means.
The two samples tested in Study 1 provide two independent tests of the undoing effect of positive emotions. Results from both tests support the undoing hypothesis, exactly replicating the preliminary findings reported by Fredrickson and Levenson (1998) . Study 1 also extends the evidence for the undoing effect of positive emotions in three important ways. First, the undoing effect occurs when the initial negative emotion generates a clear pattern of heightened sympathetic cardiovascular reactivity that is typical of anxiety, fear, and other health-damaging negative emotions. Second, the undoing effect is not limited to women, but occurs for men as well. Third, the undoing effect occurs comparably for African Americans and European Americans. Importantly, the undoing effect has now been demonstrated experimentally three times for two distinct types of positive emotion: a low-activation pleasant state of contentment and a higher-activation pleasant state of amusement. This evidence suggests that these two positive emotions—although distinct in their phenomenology and activation level—share the ability to regulate lingering negative emotional arousal. Additional studies will be needed to assess whether the undoing effect generalizes to other positive emotions, such as interest, love, pride, or excitement.
Most important, data from Study 1 indicate that the undoing effect of positive emotions is not a spurious finding, but is instead replicable and real. In addition, these data provide indirect support for Fredrickson’s broaden-and-build theory of positive emotions ( Fredrickson, 1998 ). If we take the cardiovascular activation that accompanies negative emotions to be the body’s preparation for specific action, then by quelling this activation, positive emotions may help the body move from a (no longer useful) narrow thought–action repertoire toward a broader one, allowing the individual to pursue a wider array of thoughts and actions. Moreover, laboratory evidence showing that both contentment and amusement can undo lingering negative emotional arousal provides footing for the suggestion that individuals can harness the undoing effect of positive emotions to regulate negative emotions in daily life ( Fredrickson, 2000 ).
Despite the augmented empirical support for the existence of the undoing effect, a viable alternative explanation remains. Perhaps the neutral and sad films elicit cardiovascular arousal, whereas the positive films do not. If so, instead of concluding that the positive films facilitate cardiovascular recovery, we should conclude that the neutral and sad films prolong recovery. In other words, perhaps our findings represent replacement rather than undoing: Perhaps each film simply replaces the cardiovascular reactivity produced by the speech task with its own cardiovascular signature, and the two positive films produce lower sympathetic activation than both the neutral and sad films. We now turn to Study 2 to test the viability of this replacement explanation by examining cardiovascular responses to the positive, neutral, and sad films against the backdrop of a resting baseline.
One hundred eighty-five university students (49% women) were recruited for a study on emotions through flyers and newspaper advertisements. Each was paid $30 to participate in a series of studies lasting 2 hr. Of the participants, 137 were European American (48% women) and 48 were African American (50% women). Approximately half of the participants in Study 2 later participated in Study 1, as Sample 1. For these participants, random assignment to experimental condition across Studies 1 and 2 was yoked such that no participant viewed the same film clip across the two studies.
The written and visual materials were the same as used in Study 1.
These were identical to those used in Study 1.
On-Line Affect Report
A positive–negative affect rating dial, developed by Levenson and Gottman (1983) , was used to obtain on-line, continuous reports of affect during the study. Participants manipulated a dial whose pointer moved on a 180-deg scale divided into nine divisions ranging from very negative to neutral to very positive . The dial was attached to a potentiometer in a voltage diving circuit monitored by the same computer that monitored the cardiovascular data. Participants were instructed to adjust the dial position as often as necessary so that it always reflected how positive or negative they were feeling moment-by-moment throughout the session. Validity data for this affect rating dial procedure can be found in Gottman and Levenson (1985 ; see Fredrickson & Kahneman, 1993 , for a similar on-line affect rating procedure).
Participants were tested individually by a female experimenter of their same ethnicity. The laboratory environment and introductory remarks to participants were the same as in Study 1. After physiological sensors were attached and a 5-min adaptation period, the experimenter introduced the study in more detail. Participants were told that they would view film clips that would depict either positive, negative, or neutral images and that they should watch the video monitor at all times. They were also instructed in the use of the affect rating dial. Participants were given an opportunity to practice manipulating the dial without looking down at their hand. During the actual data collection, participants were alone in the room.
Following an additional 5-min adaptation period, participants received instructions on the video monitor to “relax, and empty your mind of all thoughts, feelings and memories.” This commenced a 2-min resting baseline period, the second minute of which was used as the prefilm baseline. Immediately following this resting baseline phase, participants (blocked by sex and ethnicity) were randomly assigned to view the Waves, Puppy, Sticks, or Cry film. At the end of this film viewing trial, participants completed an Emotion Report Form to describe how they felt viewing the film clip.
Data Reduction
For each participant, we calculated mean affect rating dial reports and cardiovascular activity averaged across the 60-s prefilm baseline period and the 100-s film period. 5 To quantify participants’ responses to the films, we subtracted baseline period means from film period means. The resulting change scores for each of the four film clips are presented in Table II . For each film, and for each variable, we computed within-subject t -tests to examine whether these change scores represented significant differences from the prefilm resting baseline. The results of these tests are also reported in Table II .
Mean and Standard Deviation of Subjective and Cardiovascular Changes for the Four Film Groups in Study 2
Note . RATE: affect rating dial, ranging from 0 to 9; HR: heart rate in beats per min; FPA: finger pulse amplitude in mv; PTF: pulse transmission time to the finger in seconds; PTE: pulse transmission time to the ear in seconds; DBP: diastolic blood pressure in mmHg; SBP: systolic blood pressure in mmHg. Means in the same row with the same subscript are not significantly different at p < .05 by Tukey pairwise comparisons. Asterisks indicate changes significantly different from resting baseline measures by within-subject t -tests ( d f = 45 or 46). Values represent M ( SD ).
p < .05,
p < .01,
We wished to confirm that the four films altered subjective experiences as intended. Data from the on-line affect rating dial, presented in the first row of Table II , provided this confirmation. We explored sex and ethnicity differences by using a 2 × 2 × 4 ANOVA (Sex × Ethnicity × Film Group). Beyond the expected effect for Film Group, F (3, 169) = 51.25, p < .001, no main effects for, or interactions with, Sex or Ethnicity were significant. Moreover, Tukey pairwise comparisons (reported in row 1 of Table II ) confirmed that rating dial responses to both the Waves and the Puppy films were more positive than those to both the Sticks and the Cry films. Additionally, rating dial responses to the Cry film were more negative than those to the Sticks film.
These group differences in on-line subjective ratings were corroborated by the retrospective subjective ratings provided on the Emotion Report Forms: Tukey pairwise comparisons revealed that Waves elicited significantly more contentment ( M = 5.24, SD = 1.82) than any other clip, Puppy elicited more amusement ( M = 4.74, SD = 2.25), and Cry elicited more sadness ( M = 4.15, SD = 2.29). As in prior studies, modal emotion reports for Sticks were zero for all eight emotion terms, confirming its emotional neutrality. Differences in retrospective emotion ratings by sex and ethnicity were explored using 2 × 2 × 4 ANOVAs (Sex × Ethnicity × Film Group). Beyond the expected main effects for Film Group, only one main effect for Ethnicity emerged: Across all films, African Americans reported less contentment ( M = 2.54, SD = 2.37) than European Americans, M = 3.36, SD = 2.62, F (1, 169) = 6.23, p = .014.
Is Replacement a Viable Alternative Explanation?
Recall that the replacement explanation requires the two positive films to elicit lower sympathetic activation than both the neutral film and the sad film. Inspection of mean cardiovascular change scores in Table II suggests that the cardiovascular responses to the four films were minimal (cf., Table I showing cardiovascular responses to the anxiety-producing speech task in Study 1). In fact, no cardiovascular responses were significantly different from resting baseline levels for the Puppy or Sticks film groups. Within the Waves film group, the slight decrease in FPA was statistically significant, as was the slight increase in SBP (see Table II , column 1). Within the Cry film group, the slight decreases in HR and FPA were significant, as was the increase in SBP (see Table II , column 4).
To test for differences in cardiovascular reactivity elicited by the four films, and to explore possible differences by sex and ethnicity, we conducted a series of univariate 2 × 2 × 4 ANOVAs (Sex × Ethnicity × Film Group) on each of the six cardiovascular variables. We followed these with post hoc pairwise comparisons where indicated. We found that only FPA and SBP distinguished the Cry film from the other films, yet only for certain subgroups: Greater vasoconstriction during the Cry film was evident only among women (women: M = −0.19, SD = 0.27; men: M = −0.03, SD = 0.20, Film Group × Sex: F (3, 169) = 3.09, p = .029), and greater increases in SBP during the Cry film were strongest for women (women: M = 7.29, SD = 11.72; men: M = 1.99, SD = 5.13, Film Group × Sex: F (3, 169) = 3.64, p = .014), particularly African American women ( M = 14.96, SD = 17.65, Film Group × Sex × Ethnicity: F (3, 169) = 3.06, p = .030). The results of Tukey pairwise comparisons across Film Groups (collapsing across sex and ethnicity) are indicated in Table II . From these findings we can conclude that the sad film elicits somewhat more sympathetic reactivity than the positive and neutral films do, especially for women.
The most critical comparisons for disconfirming the replacement explanation, however, are between the neutral and positive films. Although some degree of cardiovascular reactivity was evident for one of the two positive films (Waves, see Table II , column 1), are these changes appreciably different from those produced by the neutral film? To test this, we conducted a series of 2 × 2 × 3 ANOVAs (Sex × Ethnicity × Film Group, excluding the Cry film) on the set of six cardiovascular measures. No main effects or interactions emerged as significant for any of the six variables. These null findings suggest that positive and neutral films produced statistically indistinguishable cardiovascular responses.
As expected, data from Study 2 demonstrated that althogh the selected positive and neutral films clearly differed in the subjective responses they produced, they did not differ in the cardiovascular responses they produced. In fact, cardiovascular responses to these positive and neutral films were almost nonexistent. This disconfirms the replacement hypothesis: If the positive and neutral films do not differ in their cardiovascular signatures, then the undoing effect observed in Study 1 could not result from a simple replacement process. It bears note, however, that our prediction that cardiovascular responses to positive and neutral films would not differ parallels the null hypothesis, and by consequence, we had a disproportionate chance to support it. With this limitation in mind, we consider this to be a demonstration study concerning the two positive emotion films used in this research, rather than rigorous hypothesis testing about positive emotions more generally. We conservatively conclude that—when viewed in a neutral context—the contentment-eliciting Waves clip and the amusing Puppy clip do not do much to the cardiovascular systems of those who view them.
Undoing Versus Replacement
Yet even this conservative conclusion contributes to a key issue that lingered following Fredrickson and Levenson’s initial test of the undoing effect, namely the choice between interpreting the results in terms of undoing versus replacement. An undoing explanation holds that negative emotions produce cardiovascular reactivity that may linger for variable amounts of time, and that positive emotions have a unique ability to speed the return to baseline levels of arousal. By contrast, a replacement explanation holds that the data do not reflect differential rates of recovery from negative emotion, but instead that the cardiovascular reactivity produced by the initial negative emotion has been swiftly replaced by the cardiovascular reactivity produced by the subsequent positive emotion. Such replacement implies that positive emotions quickly assume control of the cardiovascular system and substitute their own patterns of activation for that produced by the initial negative emotion.
Yet the data from Study 2 suggest that the positive and neutral films produce indistinguishable patterns of cardiovascular activation. Taking this into consideration, the replacement explanation must predict positive and neutral films to be comparable in their ability to switch to their own patterns of cardiovascular activation following negative emotional arousal, and thus comparable in their ability to facilitate returns to baseline. Moreover, to the extent that the contentment-eliciting Waves film produces slight increases in sympathetic cardiovascular activation (decreased FPA, indicative of vasoconstriction, and increased SBP), and the neutral Sticks film produced no sympathetic changes, the replacement explanation would predict slightly faster returns to baseline for the neutral film than for the contentment film. Yet data from Study 1 (and the Fredrickson–Levenson experiment) show that this is not the case. Both positive emotion films brought cardiovascular levels back to baseline faster than the emotionally neutral film. In light of data from Study 2, the replacement explanation is not sufficient to explain this pattern of results. The alternative explanation that the undoing effect operates by simple replacement can thus be ruled out.
GENERAL DISCUSSION
Taken together, Studies 1 and 2 suggest that positive emotions are unique, not in what they do to the cardiovascular system, but rather in what they can undo within this system. Put differently, the cardiovascular effects of positive emotions appear to emerge only when negative emotions have already generated cardiovascular reactivity.
Although the undoing effect of positive emotions now has demonstrated reliability, additional studies are needed to address lingering questions. For instance, what physiological mechanism mediates the effect? To date, we have explored changes in parasympathetic cardiac control (indexed by respiratory sinus arrhythmia, or RSA) both in the undoing paradigm (e.g., Study 1) as well as in comparisons across positive, neutral, and negative films (e.g., Study 2). In neither context have we observed any differences in RSA responses across experimental conditions. We thus tentatively conclude that the undoing effect of positive emotions does not occur through phasic changes in parasympathetic cardiac control. Discerning the operative underlying mechanism remains a task for future work.
Other questions linger as well. For example, is the undoing effect limited to the cardiovascular concomitants of emotions? Or, as the broaden-and-build theory would imply, can positive emotions also undo the cognitive and behavioral narrowing produced by negative emotions, and thereby restore flexible thinking and action? To the best of our knowledge, no experiments have tested this prediction directly. Even so, indirect evidence can be drawn from correlational studies. Individuals who express or report higher levels of positive emotion show more constructive and flexible coping, more abstract and long-term thinking, and greater emotional distance following stressful negative events ( Keltner & Bonanno, 1997 ; Martin, Kuiper, Olinger, & Dance, 1993 ; Lyubomirsky & Tucker, 1998 ; Stein, Folkman, Trabasso, & Richards, 1997 ).
Another question to ask is whether evidence for the undoing effect also implies that positive emotions will buffer against cardiovascular reactivity triggered by subsequently experienced negative emotions. We have explored this possibility both empirically and theoretically. At an empirical level, we have reversed the order of film viewing used in Fredrickson and Levenson’s initial test of the undoing effect, showing people first the contentment, amusement, neutral, or sad film (by random assignment), and then showing the fear clip immediately after. No evidence for a buffering effect of positive emotions emerged ( Fredrickson & Mancuso, 1996 ). At a theoretical level, we concur with other theorists that personally relevant circumstances that elicit negative emotions should reliably interrupt people’s actions and capture their attention, both psychologically and physiologically ( Levenson, 1994 ; Mandler, 1984 ; Pratto & John, 1991 ; Simon, 1967 ; Tomkins, 1995 ). We suspect that such interruption should occur no matter what people’s prior affective state. Thus, we speculate that positive emotions do not buffer against negative emotional arousal in any direct or simple way. Even so, positive emotions might, over time, bolster people’s resources for coping with circumstances that elicit negative emotions. That is, positive emotions might play an indirect buffering role by incrementing coping resources ( Folkman & Moskowitz, 2000 ). These coping resources might take the form of the physical, intellectual, or social resources described by Fredrickson’s broaden-and-build theory of positive emotions ( Fredrickson, 1998 ), the hedonic surplus described by Aspinwall (1998) , or—if the undoing effect of positive emotions were deployed regularly to speed recovery from negative emotions—the physiological toughness described by Dienstbier (1989) .
In sum, the work reported here illuminates one reason, beyond intrinsic pleasure, for the pursuit of happiness: Positive emotions help downregulate the potentially health-damaging cardiovascular reactivity that lingers following negative emotions. This effect may be especially critical for those most at risk for developing coronary heart disease. Nonetheless, the undoing effect is likely to be just one of many reasons to pursue positive emotions. The broaden-and-build theory describes many others ( Fredrickson, 1998 , in press-a , in press-b ). Chief among these is that experiences of positive emotions are thought to build individuals’ lasting personal resources. By consequence, positive emotions could be tapped to optimize people’s health and well-being ( Fredrickson, 2000 ). It appears then that we have reasons other than pure hedonism to pursue positive emotions. Evidence that positive emotions do more than simply feel good underscores the need to study them further.
ACKNOWLEDGMENTS
This research was supported by a B/START Grant awarded to the first author by the National Institute of Mental Health Grant (MH53971). I wish to extend thanks to Mark Hakim, Heather Molenda, Beth Phillips and Samantha Holmes for their assistance in collecting the data reported here. I am also grateful to Phoebe Ellsworth for her talent for creating cover stories.
Due to equipment failure, indices of blood pressure were not available for two participants in Sample 1.
Due to experimenter error, the ECG was not recorded for one participant, rendering all variables derived from ECG (i.e., HR, PTF, and PTE) missing for that participant. Due to equipment failure, indices of blood pressure were not available for two additional participants.
- Anderson NB, McNeilly M, Myers H. A biopsychosocial model of race differences in vascular reactivity. In: Blascovich J, Katkin ES, editors. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1993. pp. 83–108. [ Google Scholar ]
- Aron A, Norman CC, Aron EN, McKenna C, Heyman RE. Couple’s shared participation in novel and arousing activities and experienced relationship quality. Journal of Personality and Social Psychology. 2000;78:273–284. doi: 10.1037//0022-3514.78.2.273. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [ DOI ] [ PubMed ] [ Google Scholar ]
- Aspinwall LG. Rethinking the role of positive affect in self-regulation. Motivation and Emotion. 1998;22:1–32. [ Google Scholar ]
- Baron RA. The reduction of human aggression: A field study of the influence of incompatible reactions. Journal of Applied Social Psychology. 1976;6:260–274. [ Google Scholar ]
- Blascovich J, Katkin ES, editors. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1993. [ Google Scholar ]
- Boulton MJ, Smith PK. The social nature of play fighting and play chasing: Mechanisms and strategies underlying cooperation and compromise. In: Barkow JH, Cosmides L, Tooby J, editors. The adapted mind: Evolutionary psychology and the generation of culture. New York: Oxford University Press; 1992. pp. 429–444. [ Google Scholar ]
- Cunningham MR. What do you do when you’re happy or blue? Mood, expectancies, and behavioral interest. Motivation and Emotion. 1988;12:309–331. [ Google Scholar ]
- Diener E, Larsen RJ. The experience of emotional well-being. In: Lewis M, Haviland JM, editors. Handbook of emotions. New York: Guilford Press; 1993. [ Google Scholar ]
- Dienstbeir RA. Arousal and physiological toughness: Implications for mental and physical health. Psychological Review. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [ DOI ] [ PubMed ] [ Google Scholar ]
- Eisenberg N, Fabes RA, Losoya S. Emotional responding: Regulation, social correlates, and socialization. In: Salovey P, Sluyter DJ, editors. Emotional development and emotional intelligence: Educational implications. New York: Basicbooks; 1997. pp. 129–163. [ Google Scholar ]
- Ekman P, Friesen WV, Ancoli S. Facial signs of emotional experience. Journal of Personality and Social Psychology. 1980;39:1124–1134. [ Google Scholar ]
- Estrada CA, Isen AM, Young MJ. Positive affect facilitates integration of information and decreases anchoring in reasoning among physicians. Organizational Behavior and Human Decision Processes. 1997;72:117–135. [ Google Scholar ]
- Folkman S. Positive psychological states and coping with severe stress. Social Science Medicine. 1997;45:1207–1221. doi: 10.1016/s0277-9536(97)00040-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55:647–654. doi: 10.1037//0003-066x.55.6.647. [ DOI ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL. What good are positive emotions? Review of General Psychology. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL. Cultivating positive emotions to optimize health and well-being. Prevention and Treatment. 2000 Available on the World Wide Web: http://journals.apa.org/prevention . [ Google Scholar ]
- Fredrickson BL. The role of positive emotions in positive psychology: The broadenand-build theory of positive emotions. American Psychologist. doi: 10.1037//0003-066x.56.3.218. (in press-a) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL. Positive emotions. In: Synder CR, Lopez SJ, editors. Handbook of positive psychology. New York: Oxford University Press; (in press-b) [ Google Scholar ]
- Fredrickson BL, Kahneman D. Duration neglect in retrospective evaluations of affective episodes. Journal of Personality and Social Psychology. 1993;65:45–55. doi: 10.1037//0022-3514.65.1.45. [ DOI ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion. 1998;12:191–220. doi: 10.1080/026999398379718. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL, Mancuso RA. Testing a direct buffering effect of positive emotions. 1996 Unpublished raw data. [ Google Scholar ]
- Fredrickson BL, Maynard KE, Helms MJ, Haney TL, Siegler IC, Barefoot JC. Hostility predicts magnitude and duration of blood pressure response to anger. Journal of Behavioral Medicine. 2000;23:229–243. doi: 10.1023/a:1005596208324. [ DOI ] [ PubMed ] [ Google Scholar ]
- Frijda NH. The emotions. Cambridge: Cambridge University Press; 1986. [ Google Scholar ]
- Gottman JM, Levenson RW. A valid measure for obtaining self-report of affect. Journal of Consulting and Clinical Psychology. 1985;53:151–160. doi: 10.1037//0022-006x.53.2.151. [ DOI ] [ PubMed ] [ Google Scholar ]
- Isen AM. The influence of positive and negative affect on cognitive organization: Some implications for development. In: Stein N, Leventhal B, Trabasso T, editors. Psychological and biological approaches to emotion. Hillsdale, NJ: Erlbaum; 1990. pp. 75–94. [ Google Scholar ]
- Isen AM, Daubman KA. The influence of affect on categorization. Journal of Personality and Social Psychology. 1984;47:1206–1217. [ Google Scholar ]
- Isen AM, Daubman KA, Nowicki GP. Positive affect facilitates creative problem solving. Journal of Personality and Social Psychology. 1987;52:1122–1131. doi: 10.1037//0022-3514.52.6.1122. [ DOI ] [ PubMed ] [ Google Scholar ]
- Isen AM, Johnson MMS, Mertz E, Robinson GF. The influence of positive affect on the unusualness of word associations. Journal of Personality and Social Psychology. 1985;48:1413–1426. doi: 10.1037//0022-3514.48.6.1413. [ DOI ] [ PubMed ] [ Google Scholar ]
- Isen AM, Means B. The influence of positive affect on decision-making strategy. Social Cognition. 1983;2:18–31. [ Google Scholar ]
- Isen AM, Rosenzweig AS, Young MJ. The influence of positive affect on clinical problem solving. Medical Decision Making. 1991;11:221–227. doi: 10.1177/0272989X9101100313. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kahn BE, Isen AM. The influence of positive affect on variety-seeking among safe, enjoyable products. Journal of Consumer Research. 1993;20:257–270. [ Google Scholar ]
- Kaplan JR, Manuck SB, Williams JK, Strawn W. Psychosocial influences on atherosclerosis: Evidence for effects and mechanisms in nonhuman primates. In: Blascovich J, Katkin E, editors. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1993. [ Google Scholar ]
- Keltner D, Bonanno GA. A study of laughter and dissociation: Distinct correlates of laughter and smiling during bereavement. Journal of Personality and Social Psychology. 1997;73:687–702. doi: 10.1037//0022-3514.73.4.687. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lazarus RS. Emotion and adaptation. New York: Oxford University Press; 1991. [ Google Scholar ]
- Lee PC. Play as a means for developing relationships. In: Hinde RA, editor. Primate social relationships. Oxford: Blackwell; 1983. pp. 82–89. [ Google Scholar ]
- Leslie AM. Pretense and representation: The origins of “theory of mind.”. Psychological Review. 1987;94:412–426. [ Google Scholar ]
- Levenson RW. Emotion and the autonomic nervous system: A prospectus for research on autonomic specificity. In: Wagner HL, editor. Social psychophysiology and emotion: Theory and clinical applications. London: Wiley; 1988. pp. 17–42. [ Google Scholar ]
- Levenson RW. Human emotion: A functional view. In: Ekman P, Davidson R, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. pp. 123–126. [ Google Scholar ]
- Levenson RW, Ekman P, Friesen WV. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology. 1990;27:363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lyubomirsky S, Tucker KL. Implications of individual differences in subjective happiness for perceiving, interpreting, and thinking about life events. Motivation and Emotion. 1998;22:155–186. [ Google Scholar ]
- Mandler G. Mind and body: Psychology of emotions and stress. New York: Norton; 1984. [ Google Scholar ]
- Martin RA, Kuiper NA, Olinger J, Dance KA. Humor, coping with stress, self-concept, and psychological well-being. Humor. 1993;6:89–104. [ Google Scholar ]
- Matthews KA, Stoney CM. Influences of sex and age on cardiovascular responses during stress. Psychosomatic Medicine. 1988;50:46–56. doi: 10.1097/00006842-198801000-00006. [ DOI ] [ PubMed ] [ Google Scholar ]
- Newlin D, Levenson RW. Pre-ejection period: Measuring beta-adrenergic influences upon the heart. Psychophysiology. 1979;16:546–553. doi: 10.1111/j.1469-8986.1979.tb01519.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Panksepp J. Attention deficit hyperactivity disorders, psychostimulants, and intolerance of childhood playfulness: A tragedy in the making? Current Directions in Psychological Science. 1998;7:91–98. [ Google Scholar ]
- Pratto F, John OP. Automatic vigilance: The attention-grabbing power of negative social information. Journal of Personality and Social Psychology. 1991;61:380–391. doi: 10.1037//0022-3514.61.3.380. [ DOI ] [ PubMed ] [ Google Scholar ]
- Reed MB, Aspinwall LG. Self-affirmation reduces biased processing of health-risk information. Motivation and Emotion. 1998;22:99–132. [ Google Scholar ]
- Salovey P, Bedell BT, Detweiler JB, Mayer JD. Current directions in emotional intelligence research. In: Lewis M, Haviland-Jones JM, editors. Handbook of emotions. 2nd ed. New York: Guilford Press; 2000. pp. 504–520. [ Google Scholar ]
- Schuler JLH, O’Brien WH. Cardiovascular recovery from stress and hypertension risk factors: A meta-analytic review. Psychophysiology. 1997;34:649–659. doi: 10.1111/j.1469-8986.1997.tb02141.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Simon HA. Motivational and emotional controls of cognition. Psychological Review. 1967;74:29–39. doi: 10.1037/h0024127. [ DOI ] [ PubMed ] [ Google Scholar ]
- Solomon RL. The opponent-process theory of acquired motivation: The costs of pleasure and benefits of pain. American Psychologist. 1980;35:691–712. doi: 10.1037//0003-066x.35.8.691. [ DOI ] [ PubMed ] [ Google Scholar ]
- Stein NL, Folkman S, Trabasso T, Richards TA. Appraisal and goal processes as predictors of psychological well-being in bereaved caregivers. Journal of Personality and Social Psychology. 1997;72:872–884. doi: 10.1037//0022-3514.72.4.872. [ DOI ] [ PubMed ] [ Google Scholar ]
- Suarez EC, Williams RB. Situational determinants of cardiovascular and emotional reactivity in high and low hostile men. Psychosomatic Medicine. 1989;51:401–418. doi: 10.1097/00006842-198907000-00004. [ DOI ] [ PubMed ] [ Google Scholar ]
- Tomkins SS. Exploring affect: The selected writings of Silvan S. Tomkins. In: Demos EV, editor. New York: Cambridge University Press; 1995. [ Google Scholar ]
- Trope Y, Pomerantz EM. Resolving conflicts among self-evaluative motives: Positive experiences as a resource for overcoming defensiveness. Motivation and Emotion. 1998;22:53–72. [ Google Scholar ]
- Williams RB, Barefoot JC, Shekelle RB. The health consequences of hostility. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders. Washington, DC: Hemisphere; 1985. pp. 173–185. [ Google Scholar ]
- Wolpe J. Psychotherapy by reciprocal inhibition. Stanford: Stanford University Press; 1958. [ Google Scholar ]
- View on publisher site
- PDF (478.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM

Add to Collections

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Chapter 13: Positive Emotions
Undoing Effect of Positive Emotions
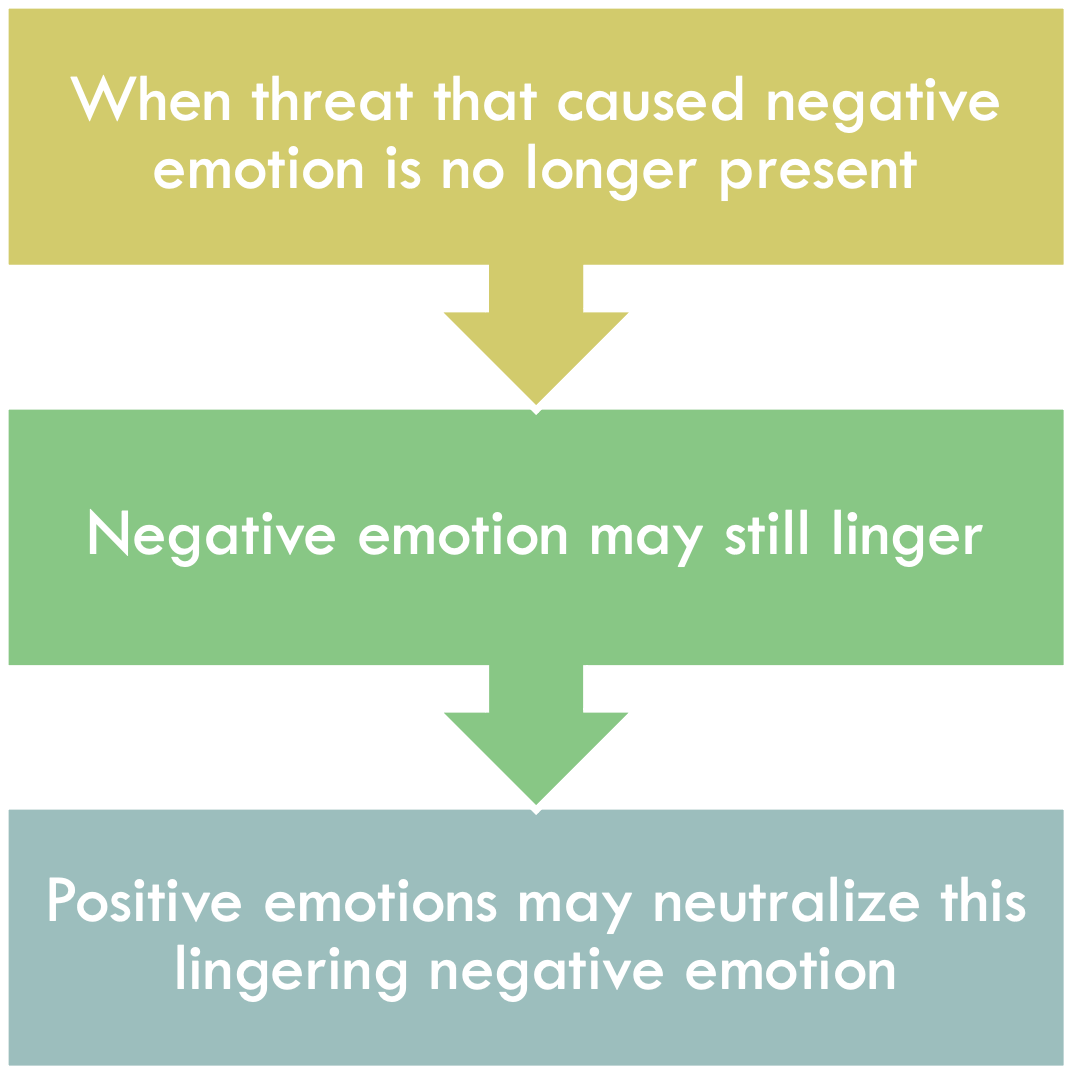
In a study that tested the undoing effect (Fredrickson et al., 2000), participants first completed baseline measures of heart rate, finger pulse, and blood pressure. Then, all participants were induced to feel a high-arousal negative emotion by telling participants they would have 60 seconds to write a 3-minute speech on a topic provided to them. After this, participants were randomly assigned to watch a film clip that elicited either amusement, contentment, neutrality, or sadness.
Remember, throughout the study physiological measures were taken. The dependent variable was cardiovascular recovery measured as the time from which it took participants physiological results to return to baseline. In Figure 21, recovery on the x-axis was measured as the time it took from the start of their assigned clip to the time when their physiology returned to baseline. Keep in mind that a short time indicates faster cardiovascular recovery.
Figure 21 Cardiovascular Recovery for Each Emotion Clip (Fredrickson et al., 2000)
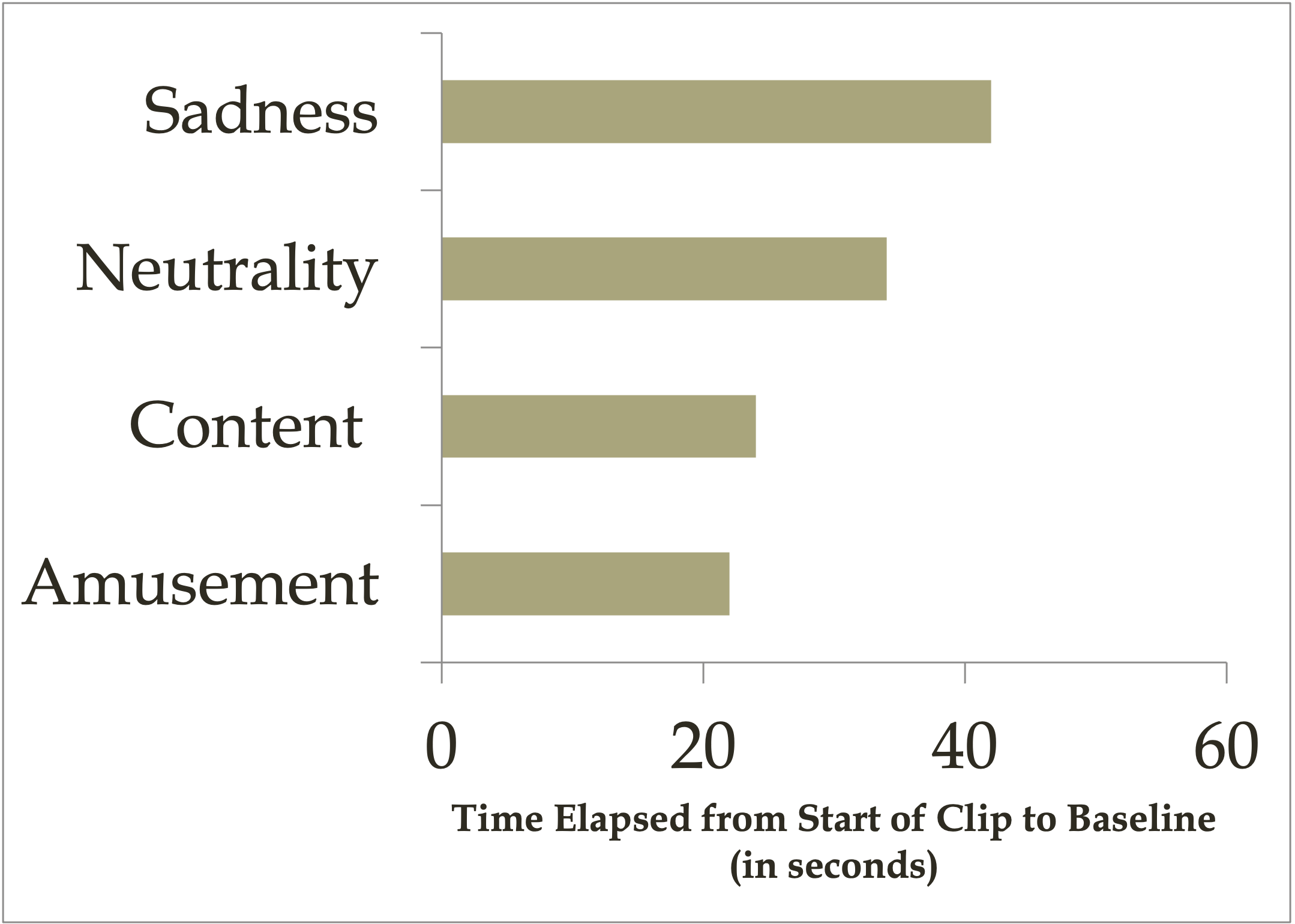
Reproduced from “The undoing effect of positive emotions,“ by B.L. Fredrickson, R.A. Mancuso, C. Branigan, and M.M. Tugade, 2000, Motivation and Emotion , 24 (4), p. 254 ( https://doi.org/10.1023/A:1010796329158 ) Copyright 2000 by Plenum.
Amusement and contentment resulted in significantly faster cardiovascular recovery than the neutral and sadness film clips. Interestingly, the neutral clip resulted in faster recovery than the sadness clip. In a follow-up study, Fredrickson and colleagues (2000) conducted the same study except that participants did not engage in the first stressor. So, participants simply viewed one of the four clips. Participants watching the sad clip exhibit more arousal than the other three conditions. Differences were not found between the positive and neutral conditions. What does this mean? This means that experiencing positive emotions does not regulate our physiology better than neutral states. Instead, experiencing a positive emotion directly after a negative emotion can help us to mitigate the negative emotional responses better than experiencing a neutral state after a negative emotion.
Undoing Effect of Positive Emotions: After a negative emotion, positive emotions help us to quickly return to baseline cardiovascular states.
One last interesting note about the undoing effect. Later work (Tugade & Fredrickson, 2004) found that the experience of positive emotions mediates the relationship between trait resilience and cardiovascular recovery (see Figure 22). In other words, people high in resilience are faster to recover from physiological arousal because resilient individuals experience more positive emotions .
Positive Emotions Mediated the Relationship between Resilience and Cardiovascular Recovery (Tugade & Fredrickson, 2004)
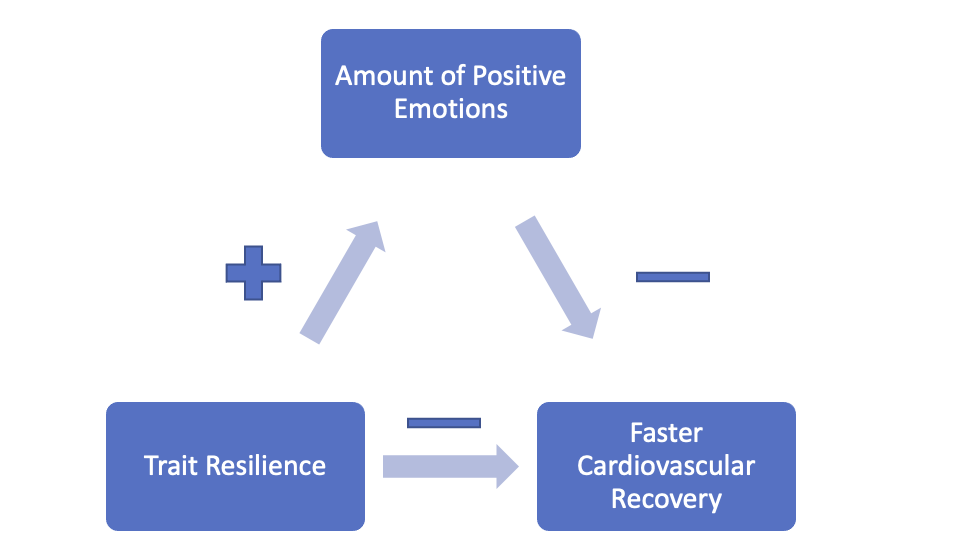
Psychology of Human Emotion: An Open Access Textbook Copyright © by Michelle Yarwood is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

I Don’t Want to Come Back Down: Undoing versus Maintaining of Reward Recovery in Older Adolescents
Kirsten e gilbert, susan nolen-hoeksema, june gruber.
- Author information
- Article notes
- Copyright and License information
Corresponding Author: Kirsten Gilbert, Department of Psychiatry, Washington University in St. Louis, 4444 Forest Park Parkway Suite 2100, St. Louis, MO 63108, [email protected] , Phone: 650-387-5991
Issue date 2016 Mar.
Adolescence is characterized by heightened and sometimes impairing reward sensitivity, yet less is known about how adolescents recover from highly arousing positive states. This is particularly important given high onset rates of psychopathology associated with reward sensitivity during late adolescence and early adulthood. The current study thus utilized a novel reward sensitivity task in order to examine potential ways in which older adolescent females (ages 18–21; N = 83) might recover from high arousal positive reward sensitive states. Participants underwent a fixed incentive reward sensitivity task and subsequently watched a neutral, sad, or a low approach-motivated positive emotional film clip during which subjective and physiological recovery was assessed. Results indicated that the positive and negative film conditions were associated with maintained physiological arousal while the neutral condition facilitated faster physiological recovery from the reward sensitivity task. Interestingly, individual differences in self-reported positive emotion during the reward task were associated with faster recovery in the neutral condition. Findings suggest elicited emotion (regardless of valence) may serve to maintain reward sensitivity while self-reported positive emotional experience may be a key ingredient facilitating physiological recovery or undoing. Understanding the nuances of reward recovery provides a critical step in understanding the etiology and persistence of reward dysregulation more generally.
Keywords: Reward, Adolescents, Emotional Recovery, Positive Emotion, Undoing hypothesis
Adolescence is characterized by heightened reward sensitivity compared with younger children and adults (e.g., Galvan, 2013 ; Steinberg, 2010 ). This elevated reward sensitivity has often been linked to seeking out increased independence and more social interactions, but is also associated with increased sensation-seeking and risk-taking behaviors (e.g., drug use, sexual promiscuity; Dahl & Gunnar, 2009 ; Galvan, 2013 ; Steinberg, 2008 ). Moreover, many forms of psychopathology that onset during adolescence and emerging adulthood are characterized by problematic reward sensitivity, including anxiety, depression, bipolar disorder, alcohol and substance abuse, eating disorders, and psychosis ( Häfner et al., 1989 ; Kessler, Chiu, Demler, & Walters, 2005 ). However, it not well understood what potential processes older adolescents can use to effectively down-regulate these high arousal positive states. Gaining a better understanding of how adolescents and emerging adults recover from reward sensitive states is thus a critical research priority (e.g., Davidson, 2015 ).
Reward sensitivity is intricately tied with positive emotional experience, as positive emotion is common elicited by the anticipation or receipt of rewarding stimuli ( Rolls, 1999 ). Specifically, reward sensitivity can be characterized as a high-arousal positive emotional state associated with increased energy mobilization and expenditure as individuals are more sensitive to pursuing and obtaining goals or rewards in their environment ( Gable & Harmon-Jones, 2011 ; Russell, 2003 ; Tsai, 2007 ). Adolescence is characterized by increases in reward sensitivity as brain systems associated with emotion and motivation develop earlier than those regions implicated in cognitive control and regulation ( Casey & Caudle, 2013 ; Luna, Paulsen, Padmanabhan, & Geier, 2013 ; Nelson, Leibenluft, McClure, & Pine, 2005 ). This imbalance in brain development leads to greater reliance on emotional systems compared with underdeveloped regulatory abilities (e.g., Casey & Caudle, 2013 ). Difficulty cognitively regulating heightened reward sensitivity peaks in mid-adolescence but continues to a lesser degree into emerging adulthood as cognitive control regions develop well into the mid-twenties ( Giedd, 2004 ).
Difficulty with reward regulation has been provided as one potential explanation for increased risk-taking behaviors ( Eaton et al., 2008 ; Galvan, 2013 ) and the high onset rates of a range of psychopathology ( Dahl & Gunnar, 2009 ; Ernst, Pine, & Hardin, 2006 ; Paus, Keshavan, & Giedd, 2008 ) in adolescence and young adulthood. In particular, adolescent females exhibit especially high rates of psychopathology compared with adolescent males, including depression ( Galambos, Leadbeater, & Barker, 2004 ), anxiety ( Lewinsohn, Gotlib, Lewinsohn, Seeley, & Allen, 1998 ), and eating disorders ( Lewinsohn, Seeley, Moerk, & Striegel-Moore, 2002 ) and these rates remain high through older adolescence and emerging adulthood. During the older adolescent period, many of these vulnerable females are also faced with new stressors such as increasing responsibilities and independence (e.g., moving away for college, a first apartment and increasing job responsibilities) and new rewards become relevant (e.g., fraternity parties, binge drinking and less parental control) while cognitive control regions are still “catching up” developmentally. Thus, it is imperative to isolate potential processes, such as reward sensitivity, that may contribute to an increased risk for psychopathology in this population. Given difficulty with traditional forms of cognitive regulation, identifying additional pathways older adolescent females may effectively regulate and recover from high arousal positive states may aid in the development of targeted intervention foci. The aim of the current study was to understand how older adolescent females may adaptively recover from heightened reward sensitive states in ways other than utilizing underdeveloped regulation or cognitive control.
Exploring Positive Emotion Recovery
Emotion recovery is defined as the rate, or degree, to which an emotional or physiological response returns to a pre-stress baseline level following a stressor ( Davidson, 2015 ; Haynes, Gannon, Orimoto, O’Brien, & Brandt, 1991 ). One well-studied strategy that facilitates emotion recovery is by means of experiencing positive emotion, which has demonstrated to aid emotional and physiological recovery via the ‘undoing hypothesis’ ( Fredrickson & Levenson, 1998 ). The undoing hypothesis suggests that positive emotions aid in recovering from, or “undoing” heightened emotional and physiological arousal often associated with negative emotion states ( Fredrickson & Levenson, 1998 ; Fredrickson, Mancuso, Branigan, & Tugade, 2000 ). Supportive evidence for this perspective has demonstrated that following a negative (i.e., sad, fearful, or anxious) affective state, subsequently eliciting and experiencing a positive (i.e., amusing or content) state leads to a faster return to pre-induction cardiovascular baseline as compared with a neutral or negative emotion state ( Fredrickson & Levenson, 1998 ; Fredrickson, Mancuso, et al., 2000 ; Tugade & Fredrickson, 2004 ).
The undoing hypothesis has provided a useful perspective on the benefits and functions of positive emotions as facilitating negative emotional recovery. Recent work has demonstrated nuances such that the undoing hypothesis is most strongly supported in the context of specific types of positive emotion, namely, low approach-motivated positive emotions such as amusement and contentment ( Gable & Harmon-Jones, 2011 ; Harmon-Jones & Gable, 2009 ). Low approach-motivated positive emotions are experienced when a goal or reward is not relevant or after a reward is obtained, so the action urge may not be to approach, while high approach-motivated positive emotions are pre-goal, reward-sensitive emotions that are associated with an urge to act or approach ( Harmon-Jones & Gable, 2009 ). Thus, positive emotion low on approach motivation (i.e., positive emotions experienced when a goal is not relevant) are purported to facilitate recovery and aid in undoing heightened arousal. However, positive emotions high on approach-motivation, such as excitement or enthusiasm, which may occur in the context of approaching a goal or reward and which may be high arousal, may thus not facilitate physiological recovery.
Interestingly, the majority of work on the undoing hypothesis to date has focused on how positive emotions facilitate recovery from negative emotions. To our knowledge it has not yet been directly tested whether low approach-motivated states might also facilitate emotion recovery from high approach-motivated rewarding stimuli as well. Given the distinctive role of low approach-motivated positive states in the undoing hypothesis, the undoing hypothesis might also apply to adaptively undoing highly physiologically activating, approach-motivated, reward sensitive emotions. Because adolescents experience heightened positive emotional arousal in the form of elevated reward sensitivity, and this heightened reactivity is also difficult to regulate ( Casey & Caudle, 2013 ; Galvan, 2013 ), the undoing hypothesis may provide one adaptive way for adolescents to recover from reward sensitive states. Stated otherwise, increasing low approach-motivated positive emotion might actually serve as a candidate way to help older adolescents recover from heightened reward sensitivity.
We also examined an alternative perspective to the undoing hypothesis which we refer to as the maintenance hypothesis. The maintenance hypothesis posits that any positive emotion, no matter the motivational intensity or valence, might lead to maintaining emotional and physiological reactivity in adolescents. Given aforementioned difficulties managing negative and positive emotional states due to ongoing neurobiological development (e.g., Casey & Caudle, 2013 ) it may be the case that adolescents are unable to reap the benefits of emotional recovery using low-approach positive emotions, but instead, any emotion may maintain or perpetuate reward-related emotional and physiological reactivity compared to experiencing no emotion. It might be, that in fact, a neutral, or low-emotional state, may lead to more undoing and faster recovery.
The Present Investigation
The present investigation examined the role of positive emotion in facilitating emotion recovery from a heightened reward sensitive state. To do so, older adolescent females underwent an experimental manipulation starting with a novel reward sensitivity task. They were then randomized to watch one of three emotional film clips: a sad, neutral, or amusing video. Subjective and physiological reactivity was measured throughout the experimental session and recovery was calculated during the emotional film clips following previously validated guidelines (e.g., Fredrickson & Levenson, 1998 ). This enabled us to examine the following aims.
The first aim was to validate a novel reward sensitivity task among older female adolescents. We hypothesized that using both monetary and social rewards in a modified and fixed incentive delay task would increase self-reported positive emotion and arousal as well as heightened physiological reactivity. This was based on previous research demonstrating that the monetary incentive delay task that the current paradigm was adapted from been associated with increased positive emotion ( Nielsen, Knutson, & Carstensen, 2008 ) and involves motivation to accrue monetary and social reward ( Knutson, Westdorp, Kaiser, & Hommer, 2000 ). Moreover, adolescents respond to social evaluation with heightened positive emotional reactivity ( Somerville, 2013 ), and when accepted by a peer, adolescent females report a boost in positive emotions and increased activation in reward-related brain regions ( Guyer, Choate, Pine, & Nelson, 2012 ). Adolescents also demonstrate increased physiological reactivity to reward ( Brenner, Beauchaine, & Sylvers, 2005 ; Richter & Gendolla, 2009 ) and heightened reward sensitivity in social contexts ( Brenner et al., 2005 ).
The second aim sought to explore the undoing hypothesis in a novel context by examining it in response to a positively valenced reward sensitivity induction in an older adolescent female population. Given that adults can effectively use low-approach positive emotional states to ‘undo’ physiological stress and arousal ( Fredrickson, et al., 2000 ), the first undoing hypothesis predicted that low approach-motivated positive emotion would facilitate effective emotion recovery (i.e., decrease in emotional and physiological intensity via return to baseline) following a reward sensitivity task as compared with a neutral or negative emotion. The second hypothesis, the maintenance hypothesis, predicted that positive emotion (and negative emotion) would not facilitate effective emotion recovery (i.e., no decrease in emotional and physiological intensity and longer or no return to baseline) following a reward sensitivity task. While we predicted any emotion to maintain reward sensitive physiological arousal, we hypothesized that the neutral, non-emotional condition would facilitate recovery.
The third aim was to examine the influence of self-reported positive emotion on reward recovery processes. Previous work suggests that increased self-reported positive emotion during stress is associated with shorter physiological recovery and better habituation to physiological stress in adolescent girls at high risk for depression ( Waugh, Muhtadie, Thompson, Joormann, & Gotlib, 2012 ). Moreover, self-reported positive emotion during a stressor mediates the relationship between resilience and faster physiological recovery in adults ( Folkman & Moskowitz, 2000 ; Waugh et al., 2012 ). We thus extended this work to examine the role of self-reported positive emotion during a reward induction on physiological recovery across the neutral and positive conditions. We hypothesized that higher self-reported positive emotion would be associated with a faster physiological return to baseline across conditions.
Participants
Participants were recruited from online postings and flyers posted in the general New Haven, CT region (N = 83). Inclusion criteria included community sample females between the ages of 18 and 21 ( M = 19.68, SD = 1.13). Only females were recruited because male and female adolescents differ in both behavioral and self-reported sensation seeking and risk taking behaviors, with males displaying higher sensation seeking compared with females ( Steinberg et al., 2008 ). Participants ( N = 83) were Caucasian (44.6%), Asian (19.3%), African American (15.7%), Hispanic (9.6%), and Other (10.8%) ethnicities. Participants in each condition did not differ on age, F (2,80) = 0.84, p = .44 or ethnicity χ 2 (8, N = 83) = 1.94, p = 0.98.
Reward Sensitivity Task
Participants completed a novel ‘Money Winning Task,’ to elicit elevated reward sensitivity. The task was a modified monetary incentive delay (MID) task ( Knutson, Fong, Bennett, Adams, & Hommer, 2003 ), a reaction time task during which participants have to respond quickly to cued targets in order to gain money. The current task was modified from the original MID task to exclude a ‘loss’ condition to isolate anticipatory and consummatory reward winning. Instructions stated, “If you are fast enough to hit a target, you can win different amounts of money.” During the task, each trial presented a cue on the screen indicating an amount of money ($0.00, $.50, or $1.00) that could be gained on that trial (anticipatory phase). Following a short delay (2 seconds), a target appeared on the screen and the participant responded as quickly as possible by pressing a computer key. The screen then flashed whether or not the participant responded fast enough to win the previously cued amount of money and also listed the total amount of money won from previous trials. Participants completed two pre-determined and standardized blocks of trials where more money was won than lost at two-thirds win to lose ratio (for a total of 21 trials across the two blocks, n = 14 or 66% resulted in wins). The blocks were predetermined so that the same amount of money was won by all participants. The current MID task also used a lengthened delay between cue and target to increase anticipatory gain of reward, similar to a behavioral version of this task that effectively increased subjective positive arousal during anticipation of winning in young adults ( Nielsen et al., 2008 ).
In order to further increase reward sensitivity, a social evaluative component was included and predetermined positive feedback was provided, given that social evaluation is heightened during adolescence and it increases positive emotion and reward-related reactivity, especially in female adolescents ( Brenner et al., 2005 ; Guyer et al., 2012 ; Somerville, 2013 ). Prior to task start, adolescents were instructed that if they perform better than 75% of their peers on the first block of trials, they could complete a second round of the game and have a chance to win more money. Following the first block of trials, a screen appeared stating that the computer was tabulating scores to compare the adolescent’s score with those of her peers. After 15 seconds to allow for social-evaluative reward anticipation, all adolescents read, “Congratulations, you have performed in the top 25% and you can now complete a second round to earn more money! Good luck!” A second block of predetermined trials then commenced, and across both blocks all adolescents won $5.00.
Self-reported emotion
Participants assessed their emotion and arousal over the course of the experimental session using the Self-Assessment Manikin (SAM; Bradley & Lang, 1994 ) and individual emotion items. The SAM is a quick, non-verbal 9 point rating of emotion that consists of graphic pictures of valence and arousal (a third item assessing dominance was not used). The valence figures start at a frowning (negative face), include a neutral face and end in a smiling (positive face). The arousal figures consist of a sleeping figure (not-aroused) to a figure that appears to be moving uncontrollably (highly aroused). Prior to experimental procedure starting, participants were provided a verbal explanation from the experimenter about how to use the SAM and were given a chance to ask questions. The SAM was assessed at baseline, during the reward induction and following the film clip. Additionally, because the negative film clip has been validated to induce sadness and because the positive emotion film clip being used has been validated to increase amusement ( Rottenberg, Ray, & Gross, 2007 ), participants completed ratings of written items of ‘sad’ and ‘amused’ using a Likert scale from 1 ( very slightly or not at all ) to 5 ( extremely ). These emotion word items were not pictoral and were only included at baseline and following the film clip.
Psychophysiological response
Continuous recordings of physiological activity were measured at a sampling rate of 1000 Hz, recorded using a Biopac MP150 system and analyzed with ACQKnowledge 4.1 (Biopac Systems Inc, Santa Barbara, CA). A transistor-transistor logic (TTL) digital signal enabled the synchronization of physiological data with the onset and offset of the different experimental periods. Artifacts and recording errors were corrected offline and values more or less than 3.0 standard deviations were deemed outliers and Winsorized (i.e., reassigned a value at the next highest or lowest value that is not an outlier).
Heart rate (HR)
An EKG signal was recorded by applying two pre-gelled Ag-AgCl disposable snap electrodes in a modified Lead II configuration. A Biopac ECG100C amplifier with a high pass filter of .5 Hz measured HR by importing the EKG signal into QRSTool ( Allen, Chambers, & Towers, 2007 ) and an IBI series was created by applying an automatic R-peak detector. This series was then corrected manually and imported into CmetX ( Allen et al., 2007 ) for calculation of mean HR, in beats per minute, for the individual experimental periods.
Respiratory sinus arrhythmia (RSA)
RSA is the rhythm created by the oscillation in heart rate as a result of respiration ( Bernardi, Porta, Gabutti, Spicuzza, & Sleight, 2001 ; Bernston, Cacioppo, & Quigley, 1993 ). It was obtained using the Biopac ECG100C amplifier and a respiration signal using Biopac’s RSP100C respiration module with a high pass filter of .05 Hz and a low pass filter of 1 Hz. Using AcqKnowledge 4.1, RSA was calculated using the Grossman peak-valley method, which calculates the distance between the shortest and longest R-R interval for each breath. Higher RSA values are associated with higher parasympathetic influence.
Pre-ejection period (PEP)
PEP is a measure of sympathetic arousal that has been implicated in reward ( Brenner et al., 2005 ; Sherwood et al., 1990 ). PEP is the systolic time interval starting from the Q in the QRS complex to the cardiac ejection when the aortic valve is opened and it measures myocardial contractility. Impedance cardiography (Z) was measured using the Biopac NICO 100C module set at 50 kHz frequency with a low pass filter of 10 Hz and with four Biopac strip-electrodes: two parallel electrodes on the neck and two on the lower back. PEP was calculated using the derivative of Z, dz/dt in conjunction with the EKG signal and cleaned using motion artifact removal, the adaptive matching function, and interpolation of out-of-range values in AcqKnowledge 4.1. PEP is measured in seconds and smaller values of PEP indicate higher ventricular contractibility and increased sympathetic innervation on the heart.
Finger pulse amplitude (FPA)
Finger pulse amplitude measures the amount of blood pumped in the tip of the finger by measuring from the trough to the peak of the finger pulse. FPA is an index of peripheral vasoconstriction, and increased vasoconstriction in the fingertip (i.e., less blood flow to the fingertips) is a result of cardiovascular sympathetic activation. A plethysmograph was applied to the distal phalanges of the first finger of the non-dominant hand to measure finger pulse and was calculated using a 100C PPG amplifier set to AC coupling and with a low pass of 3.0 Hz and a high pass of 0.5 Hz. Using AcqKnowledge 4.1, data were resampled offline to 250 Hz and the trough-to-peak amplitude was calculated for each finger pulse, which was measured in millivolts (mv).
All participants first completed informed consent and self-report measures. Next, an experimenter explained and attached non-invasive physiological sensors to the participant for the experimental part of the study and explained how to use the SAM. Sitting in front of a computer, participants completed a five-minute adaptation period during which the participant remained quiet and still and physiological recordings were obtained. Following the adaptation, physiological recordings were obtained during a 90 second resting baseline during which participants were instructed to remain seated. Immediately following, participants current emotional state was assessed using the SAM valence and arousal measures and the positive (amused) and negative (sad) emotional words.
Participants then played the “Money Winning Task” in order to induce heightened reward sensitivity. The reward induction took approximately five minutes and physiological recordings were obtained throughout the entire task. During the second block of trials, participants were prompted with the two item SAM non-verbal mood rating assessment in between trials to assess current emotional state. The emotional word items (amused and sad) were not included at this second rating as to provide minimal disturbance in emotional and physiological responding during the reward induction ( Lieberman et al., 2007 ; Taylor, Phan, Decker, & Liberzon, 2003 ). Immediately following completion of the reward induction, all adolescents were randomized to watch either a negative ( n = 27), neutral ( n = 29) or positive ( n = 27) film clip.
Three previously validated film clips were utilized to induce specific emotional states ( Rottenberg et al., 2007 ). A 171 second clip from “The Champ” ( Lovell & Zeffirelli, 1979 ) depicting a boy crying as he watches his father die was used for the sad film, that has been validated to show an increase in sadness ( Gross & Levenson, 1995 ; Rottenberg et al., 2007 ) and has been used as a comparison to positive and neutral film clips in previous “undoing” studies ( Fredrickson, Mancuso, et al., 2000 ). Sadness is also a low approach-motivated negative emotion ( Gable & Harmon-Jones, 2010 ). The positive film clip was drawn from the television show “Whose Line is it Anyway?” This 223 second film clip depicts a stand-up comedian creating an ice cream sundae and it has been validated to elicit high levels of amusement ( Rottenberg et al., 2007 ). Amusement is a low approach-motivated positive emotion that is not associated with motivation towards a goal or reward and has been reliably used to test the undoing hypothesis ( Fredrickson, Mancuso, et al., 2000 ). A neutral emotional state was induced by showing an instructional video on how to apply wallpaper for 200 seconds ( Curby, Johnson, & Tyson, 2012 ). Physiological recordings were obtained for 171 seconds, the length of the shortest film clip. Immediately following completion of the clips, a third SAM and individual emotion rating assessed how participants felt during the assigned film clip. Participants then completed other tasks not related to the current study and then watched a 60-second positive film clip of puppies and kittens to effectively bring participants back to a stable emotional baseline (Joormann, Gilbert, & Gotlib, 2010). Finally, participants were debriefed and paid $20 for their time.
Data Analytic Strategy
We tested Aim 1 by validating changes in subjective and cardiovascular functioning from baseline to during the novel reward sensitivity task. Second, we conducted a manipulation check to confirm that each film induced the subjective emotional state intended. To assess Aim 1 and the manipulation checks, we employed repeated measures MANOVA for each domain of change (subjective and physiological; see Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005 ; Rottenberg, Kasch, Gross, & Gotlib, 2002 ) with Time as the within subjects variable and Condition as the between subjects variable and using Pillai’s Trace measures of significance. To test Aim 2, we performed a priori planned contrasts predicting that the duration of time to recover from the cardiovascular reactivity elicited from the reward induction to baseline during the positive emotional film clip to either be 1) faster than the duration of time to recover from cardiovascular reactivity elicited from the reward induction to baseline during the neutral emotional film clip (undoing) or 2) slower than the duration of time to recover from the cardiovascular reactivity elicited from the reward induction to baseline during the neutral film clip, but not different than the negative film clip (maintenance). For Aim 3, we performed a hierarchal linear regression to examine the role of self-reported positive emotion on emotion recovery. Missing data were deleted listwise and multicollinearity diagnostics showed tolerance statistics below standards. Block 1 included the centered independent factor of subjective emotion during the reward induction, Block 2 included dummy coded condition (neutral versus positive), and Block 3 included the interaction between subjective emotion and condition. We tested significant interactions using simple slopes analyses. Two separate regressions were run for each of the recovery measures.
For Aim 1, we assessed if there were any condition differences during the adaptation and baseline measurements using a one-way ANOVA. No differences emerged for any subjective emotion or physiological indices ( p ’s > .05), except for RSA at baseline, F (2,79) = 3.41, p = .04, η p 2 = 0.04. However, follow up Tukey or Bonferroni post hoc tests revealed no condition differences in RSA at baseline, and given there were also no RSA differences during adaptation, we continued as planned for Aim 1, assessing subjective and physiological responses to the reward induction. The MANOVA conducted on subjective experience included SAM valence and arousal as dependent variables, Time (baseline to two-thirds of the way through the reward induction) as the within-subjects variable, and Condition (positive, negative, neutral) as the between subjects variable (see Table 1 ). This MANVOA yielded a significant main effect of Time, F (2, 78) = 39.92, p = .00, η p 2 = 0.51, but no main effect of Condition, F (4, 158) = 0.12, p = .98, η p 2 = 0.00 and no Time × Condition interaction, F (4, 158) = 1.38, p = .25, η p 2 = 0.03. Follow up univariate repeated measures ANOVA’s demonstrated that for the main effect of Time, self-reported positive emotion (SAM valence), F (1, 79) = 4.93 p = .03, η p 2 = 0.06 and self-reported arousal (SAM arousal), F (1, 79) = 80.86, p = .00, η p 2 = 0.51, increased from baseline to mid-reward induction. For physiological variables, we used the same analytic strategy of repeated measures MANOVA to assess physiological dependent variables of HR, FPA, PEP, and RSA from the 90 second baseline to the 90 second reward induction (see Table 1 ). The MANOVA revealed a main effect of Time, F (4, 60) = 15.32, p = .00, η p 2 = 0.51, but no main effect of Condition, F (8, 122) = 1.79, p = .09, η p 2 = 0.11, and no Time × Condition interaction, F (8, 122) = 0.60, p = .77, η p 2 = 0.04. Follow up univariate repeated measures ANOVA revealed that from baseline to the reward induction, HR significantly increased, F (1, 73) = 10.21, p = .00, η p 2 = 0.12, RSA significantly increased, F (1, 76) = 10.99, p = .00, η p 2 = 0.13, PEP significantly decreased, F (1, 74) = 5.88, p = .02, η p 2 = 0.07, FPA significantly decreased, F (1, 72) = 25.09, p = .00, η p 2 = 0.26. When asked following the experiment whether they could tell that the task was predetermined, no participants spontaneously indicated knowledge of the experiment being fixed, however once the experimenter debriefed participants, four (5%) endorsed knowing the monetary portion and two (2%) assumed the social comparison were predetermined. When these participants were removed and analyses re-run, subjective and physiological results did not differ and thus these participants were kept in all subsequent analyses. Together, the reward induction increased sympathetic (FPA, PEP) and cardiovascular arousal (HR) arousal and also increased parasympathetic responding (RSA).
Means, Standard Deviations and 95% Confidence Intervals of Baseline and Reward Induction task across Conditions.
Note: Values represented are Means and Standard Deviation in parentheses; SAM Valence higher scores indicate lower mood; SAM Arousal higher scores indicate less arousal; HR = Heart rate in beats per min; FPA = Finger Pulse Amplitude in mv; PEP = Pre-ejection Period in seconds; RSA =Respiratory Sinus Arrhythmia as natural log of variance of interbeat interval time series.
p < .05;
p < .01,
To test whether each emotional film clip elicited the subjective emotional experience desired, we completed a repeated measures MANOVA of SAM valence, SAM arousal, and individual emotion ratings of sadness and amusement from baseline to immediately following the film clips. If multivariate effects were detected, we followed up these findings with univariate repeated measures ANOVA. If significant univariate interactions were found (Time × Condition), we completed paired t -tests as well as a one-way ANOVA of group differences at the second time point. MANOVA results indicated a main effect of Time, F (4, 76) = 8.20, p = .00, η p 2 = 0.30, a main effect of Condition, F (8, 154) = 12.25, p = .00, η p 2 = 0.39, and a Time × Condition interaction, F (8, 154) = 18.04, p = .00, η p 2 = 0.48. Univariate follow up tests revealed significant Time × Condition interactions for SAM valence, F (2, 79) = 54.44, p = .00, η p 2 = 0.56, SAM arousal, F (2, 79) = 16.52, p = .00, η p 2 = 0.16, sadness, F (2, 79) = 12.07, p = .00, η p 2 = 0.50, and amusement, F (2, 79) = 18.84, p = .00, η p 2 = 0.45 (see Figure 1 ).
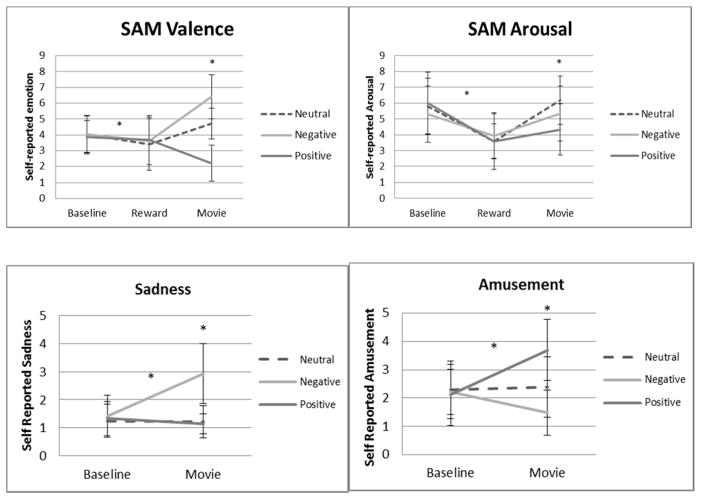
Mean Subjective Response to Reward task and Movie Clip in the Neutral, Positive, and Negative Conditions.
Note. Error bars represent standard deviations; Higher values for SAM valence indicate higher negative emotion; Higher values for SAM arousal indicate a less aroused (more calm) state; Asterisk between conditions denotes significant main effect of Time; Asterisk over a time point denotes significant interaction demonstrating differences at that time point. * p < .05.
Follow up t -tests for each group separately revealed from baseline to post-movie, SAM valence ratings became more negative in the neutral condition, paired t (27) = −3.40, p = .00 and the sad condition, paired t (26) = −6.40, p = .00, and more positive in the amusement condition, paired t (26) = 6.36, p = .00. Groups significantly differed from each other following the movie, F (2,79) = 87.93, p = .00, η p 2 = 0.69, and Bonferroni comparisons revealed that all three groups differed from each other with the amusement condition reporting the highest positive emotion ( M = 2.22, SD = 1.12), followed by the neutral condition, ( M = 4.71, SD = 0.98), and then the sad condition ( M = 6.41, SD = 1.39). For SAM arousal, follow-up paired t- tests revealed that arousal did not change from baseline to following the movie for the neutral condition, paired t (27) = −1.35, p = .19 or in the sad condition, paired t (26) = −0.08, p = .94, but arousal subjectively increased in the amusement condition, paired t (26) = 4.14, p = .00. Examining arousal following the movie, the groups did significantly differ, F (2,79) = 8.84, p = .00, η p 2 = 0.18 with the amusement condition demonstrating higher subjective arousal ( M = 4.33, SD = 1.62) compared with the neutral condition ( M = 6.19, SD = 1.54) while the negative condition did not significantly differ from either group ( M = 5.33, SD = 1.73). For individual emotion ratings of sadness, follow-up paired t -tests revealed that neither the neutral condition, paired t (27) = 0.37, p = .71 nor the amusement condition, paired t (26) = 1.99, p = .06 changed in self-reported sadness, while the sad condition increased, paired t(26) = −6.84, p = .00. Moreover, groups differed in sadness following the movie, F (2,79) = 52.10, p = .00, η p 2 = 0.57 as the sad condition ( M = 2.93, SD = 1.07) reported significantly higher sadness compared with the neutral ( M = 1.21, SD = 0.57) and amusement condition ( M = 1.15, SD = 0.36). The neutral and amusement groups did not differ. For emotion ratings of amusement, follow up paired t -tests demonstrated that the neutral condition did not change in amusement, paired t(27) = −0.60, p = .56 , but the sad condition decreased in amusement, paired t(26) = 3.92, p = .00, while the amusement condition increased in amusement, paired t(26) = −6.46, p = .00. The groups differed on amusement following the movie, F (2,79) = 34.62, p = .00, η p 2 = 0.47, and all three groups significantly differed from each other with the amusement condition endorsing the highest amusement ( M = 3.70, SD = 1.07), followed by the neutral, ( M = 2.39, SD = 1.07) and then the sad ( M = 1.48, SD = 0.80) conditions.
For aim 2, we quantified physiological recovery as the time, in seconds, taken for the individual physiological response indices to return to the participants’ own baseline confidence interval for 5 of 6 consecutive seconds. We created a baseline confidence interval by adding and subtracting one standard deviation from the mean measure of response during the 90 second resting baseline period as has been previously been done to assess physiological recovery and the undoing hypothesis ( Fredrickson & Levenson, 1998 ; Fredrickson, Mancuso, et al., 2000 ). We utilized second by second data and recovery times three plus or minus standard deviations were deemed outliers and were Winsorized prior to analysis. We also included a second measure of recovery that summed the total number of seconds each participant’s physiological score remained in the baseline confidence interval (“baseline CI”) during the entire emotional film clip. We included this measure because our first measure did not account for the possibility that once recovery is reached, participants might fluctuate out of the baseline CI recovery zone and thus, were never fully recovered. For both recovery measures, indices of cardiovascular recovery (HR, FPA, and PEP) were assessed by running two planned contrasts, 1) comparing the neutral and positive condition and 2) comparing the neutral condition with the positive and negative conditions combined.
For the first measure of recovery assessing time to recover, contrasts revealed no group differences in recovery in HR between the neutral ( M = 15.10, SD = 16.06; 95% CI [8.88 – 21.34]) and positive ( M = 20.20, SD = 24.39; 95% CI [10.13 – 30.26]) condition, t (75) = 0.82, p = .42, d = .10 nor any group differences in recovery between the neutral and combined negative ( M = 22.64, SD = 26.99; 95% CI [11.50 – 33.78]) and positive conditions, t (75) = 1.18, p = .24, d = .27. Similar results were found for contrasts of FPA between the neutral and positive condition, t (48) = −1.37, p = .18, d = −.40, and between the neutral and combined negative and positive condition, t (48) = −1.14, p = .26, d = .33, and for PEP, in the neutral versus positive, t (72) = 1.11, p = .27, d = .26, and the neutral versus combined negative and positive t (72) = 0.48, p = .64, d = .11.
For the second recovery measure assessing the total time spent in the baseline confidence interval (“baseline CI”), there was a group difference in HR between the neutral and positive condition, t (77) = −2.83, p = .01, d = .65, and a group difference between the neutral compared with the combined negative and positive condition, t (77) = −2.29, p = .03, d = −.52. The neutral condition ( M = 106.79, SD = 29.97; 95 % CI [95.17 – 118.40]) spent more time in the HR baseline CI compared with the positive ( M = 81.50, SD = 31.58; 95 % CI [68.74 – 94.25]) and positive and negative ( M = 96.81, SD = 36.78; 95% CI [81.95 – 111.66]) combined group. No significant group differences emerged for PEP when comparing the neutral to the positive condition, t (75) = −0.13, p = .90, d = −.03, or the neutral to the combined positive and negative condition, t (75) = −0.50, p = .62, d = −.12, or for FPA in either contrast: neutral versus positive, t (65) = −0.67, p = .51, d = −.17, or neutral versus combined negative and positive, t (65) = −1.18, p = .24, d = −.29.
For Aim 3, we examined the extent to which self-reported positive emotion experienced during the reward induction influenced our two physiological recovery measures of HR during the positive and neutral conditions. For HR recovery, subjective emotion during the reward induction entered into Model 1 and Condition added in Model 2 were not significant predictors of HR recovery (see Table 2 ). When the interaction between mood and condition were entered in Model 3, the interaction predicted HR recovery (Model 3: F (3,49) = 3.42, R 2 = 0.17, ΔR 2 = 0.16; Condition by Mood: β = 0.58, p = .004). To interpret this interaction, simple slopes were tested and both the neutral and positive conditions revealed significant associations. In the neutral condition a more positive mood was associated with faster HR recovery ( b = 5.16; SE = 2.23, t = 2.27, p = .027), while in the positive condition, a more positive mood was associated with slower HR recovery ( b = −4.90, SE = 2.40, t = −2.04, p = .047) (See Figure 2a ). For Time in the baseline CI, subjective mood in Model 1 was not a significant predictor, although Model 2 was significant when group was entered, (Model 2: F (2,51) = 4.61, R 2 = 0.15, ΔR 2 = 0.14; Condition: β = 0.38, p = .005). When the interaction was entered into Model 3, it was also significant (Model 3: F (3,50) = 5.72, R 2 = 0.26, ΔR 2 = 0.10; Condition by Mood: β = −0.47, p = .01). Similarly, simple slopes testing the interaction revealed that a more positive mood was associated with longer time spent in baseline CI for the neutral condition ( b = −7.65, SE = 3.49, t = −2.19, p = .03), however no significant association was found in the positive condition ( b = 5.71, SE = 3.68, t = 1.55, p = .13 (See Figure 2b ).
Hierarchical Multiple Regression Analyses Predicting Heart Rate Recovery Measures From Subjective Mood and Arousal during the Reward Induction
Note. Mood = Subjective SAM Mood rating during reward induction; Condition: Amusement = 0, Neutral = 1.
p < .01.
Simple Slopes for Subjective Mood on Recovery Indices.
Note: PA = state positive affect during reward induction. HR Recovery = Time in seconds until HR is recovered. * p < .05
Note: PA = state positive affect during reward induction. Time in CI = Total Time Spent in Baseline Confidence Interval. * p < .05
The present investigation assessed the validity of a novel reward sensitivity induction in an older adolescent female population and examined recovery from a heightened reward sensitivity state, a common emotional state in adolescence and emerging adulthood that can be difficult to effectively manage and often leads to maladaptive outcomes (e.g., Dahl & Gunnar, 2009 ; Galvan, 2013 ; Steinberg et al., 2008 ). Specifically, we examined how low approach-motivated positive emotion might serve as a particularly effective down-regulator of these heightened high approach-motivated reward sensitive states, and tested two competing hypotheses regarding the extent to which positive emotional states may foster recovery (undoing hypothesis) versus maintaining the status quo (maintenance hypothesis). This study is novel insofar as it is the first time that the undoing hypothesis and physiological recovery from a positive emotional reward sensitive states has been tested during older adolescence, a critical developmental time when learning effective strategies to down-regulate reward sensitivity is particularly important.
Results indicated partial support for the maintenance hypothesis insofar as low approach-motivated positive emotions did not lead to faster physiological emotion recovery from reward sensitive states; but by contrast, the positive (and negative) conditions were associated with maintained physiological heart rate reactivity during the recovery period. Moreover, higher subjective positive emotion during the positive emotion condition was associated with perpetuated physiological reactivity. Older adolescents may exhibit particular difficulty recovering from these reward-related positive emotional states given heightened reward sensitivity and emotional reactivity characteristic of this developmental period (e.g., Galvan, 2013 ). However, it should be noted that some conditional support also emerged for the undoing hypothesis: when not induced into an emotional state (i.e., in the neutral low-emotional condition), subjective positive emotion during the reward sensitivity induction led to faster physiological recovery. Critical insights provided for the first time by this research are that induced low-approach positive emotion following the reward induction maintains heightened reward sensitive reactivity, but that individual differences in subjectively reported positive emotion experienced during (rather than following ) the heightened reward sensitivity induction aid in faster recovery.
The first aim of this study was to test the validity of a novel reward sensitivity induction in an older adolescent female population. This was a critical methodological step to ensure that a uniform reward sensitivity state was induced across all participants. Consistent with our hypotheses and a similar to a modified version of this task for adults ( Nielsen et al., 2008 ), the reward sensitivity induction successfully increased subjective positive emotion and arousal in older adolescent females. Physiologically, the reward induction resulted in increased heart rate, elevations in indicators of sympathetic activity (i.e., FPA and PEP) as well as indicators of parasympathetic activity (i.e., RSA). This suggests that the task elicited both physiological correlates that have been associated with positive emotion (RSA; Kogan, Gruber, Shallcross, Ford, & Mauss, 2013 ; Kok & Fredrickson, 2010 ; Oveis et al., 2009 ), as well as physiological correlates of increased sympathetic arousal associated with reward, behavioral activation, and elevated goal-striving and attainment ( Brenner et al., 2005 ; Kreibig, Gendolla, & Scherer, 2010 ; Richter & Gendolla, 2009 ). This heightened sympathetic arousal leads to body mobilization and preparedness to act ( Levenson, 1994 ), which would be required during high approach-motivated reward sensitivity positive emotional states. The current reward induction was also novel in that it is one of the few (for neuroimaging paradigm see Forbes et al., 2009 ) to operationalize a heightened subjective and physiological reward sensitive state as an independent variable that did not depend on participant performance. Most tasks used to activate reward sensitivity are behavioral tasks that participants ‘play’ to win money or social feedback, such that performance on the task dictates how much reward is obtained (e.g., Cauffman et al., 2010 ; Galvan, 2006 ; Rademacher et al., 2010 ; Rao et al., 2011 ; Vaidya, Knutson, O’Leary, Block, & Magnotta, 2013 ). The current induction enables theoretical disentangling of reward sensitivity that is distinct from performance or success on the task itself.
The second aim of the study sought to test the viability of the undoing hypothesis of positive emotion compared with the maintenance hypothesis when recovering from heightened reward sensitivity. Results indicated some support for the maintenance hypothesis as the neutral condition demonstrated significantly more total time spent in the baseline confidence interval for heart rate during the recovery film clip compared with the negative or positive emotional conditions. This suggests that positive emotion did not aid in undoing heart rate, but in fact, may have served to maintain or perpetuate elevated heart rate during a recovery period. This is in contrast to work suggesting that (low approach-motivated) positive emotion aids in faster physiological recovery from various forms of heightened emotional arousal, including stress-induced anxiety, anger, fear, and sadness ( Fredrickson & Levenson, 1998 ; Fredrickson, Mancuso, et al., 2000 ; Fredrickson, Maynard, et al., 2000 ).
Several possible explanations may help understand these findings. First, the present study is the first to focus on recovery from heightened positive reward-sensitive states. Although we did switch motivational intensity (from high to low-approach) to facilitate recovery, the valence remained constant (positive emotion in both cases). Although the current study tested recovery when valence was switched by using a low approach-motivated negative emotion condition to recover from the positive reward sensitivity induction, it may be the case that the necessary ingredients for recovery from reward-focused positive emotional states are fundamentally different than those that facilitate recovery from stress or negative emotional states. Interestingly, our findings did suggest that even in the presence of an induced sad mood (a switch in valence from positive to negative during recovery), which is often associated with decreased physiological responding ( Kreibig, 2010 ), heightened physiological reactivity associated with reward reactivity was maintained. Second, although our population was on the older end of adolescence and thus peak reward-reactivity has already passed (e.g., Steinberg, 2010 ), given the ongoing development of prefrontal regions through the mid-twenties ( Giedd, 2004 ), imbalanced neurobiological development may have impeded the ability to capitalize on the adaptive outcomes of low approach-motivated positive emotions. Specifically, the heightened physiological responding to the reward sensitivity task might have been of great enough intensity to carry over into the emotional conditions.
No matter what the mechanism, continually experiencing this perpetuated activated state may lead to increased vulnerability for making risky decisions and onset of psychopathology. This heightened approach-motivated and physiologically activated state might contribute to a form of allostatic load, the notion that the body experiences ‘wear and tear’ from chronic heightened physiological arousal ( McEwen, 1998 ; Seeman, McEwen, Rowe, & Singer, 2001 ). The effects of this allostastic load may be a mechanism that puts older adolescents at greater risk for developing psychopathology characterized by heightened approach-motivated reward dysregulation (e.g., Gilbert, 2012 ). Given that an inability to physiologically recover from heightened reward sensitivity may lead to increased vulnerability to developing disorders characterized by reward-dysregulation, future work would benefit from investigating the repeated reactivity to, recovery from, and habituation to reward sensitivity in adolescents both experimentally (e.g., Waugh et al., 2012 ) and longitudinally.
These findings also indicate that distracting with neutral information may help to adaptively regulate heightened reward sensitivity. When reward saliency is high, trying to distract with another positive emotion (e.g., thinking about a funny incident with friends) or even a negative emotion (e.g., thinking about missing out on the last party) as a way to regulate, may perpetuate emotional arousal, while thinking about neutral distracting material (i.e., thinking about tomorrows weather), might help individuals better downregulate reward sensitivity and associated physiological activation. Distraction is an adaptive emotion regulation strategy in certain contexts (e.g., Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008 ; Sheppes, Catran, & Meiran, 2009 ), and when distracting with neutral material, it may also be an adaptive way for older adolescents to downregulate heightened reward sensitivity. It should be noted that we did not include any measure of task engagement while watching the film clips and so another explanation might be that the individuals in the neutral condition were daydreaming or possibly engaging in self-relevant processing or mind-wandering rather than actively distracting. Given mind-wandering is commonly reported ( Killingsworth & Gilbert, 2010 ), this possible passive daydreaming or mind-wandering might have led to disengagement from the positive emotions elicited by the reward and potentially a differential decrease in physiological responding. Studying the mechanisms and specific processes underlying the faster recovery in the neutral condition will be important to assess in future work so as to better understand the various adaptive and maladaptive ways to downregulate heightened reward sensitivity.
The third aim examined how self-reported positive emotion might influence physiological recovery from reward sensitivity. Results differed by condition, such that only in the neutral condition, higher subjective positive emotion during the reward induction was associated with faster heart rate recovery and more time spent in the baseline heart rate confidence interval. Higher state positive emotion during a stressor has been shown to lead to better coping, faster physiological recovery from the stressor, and better habituation to stress ( Folkman & Moskowitz, 2000 ; Waugh et al., 2012 ). Our finding provides conditional support of the undoing hypothesis: that when no other emotion is experienced following the reward induction, the ability to experience greater positive emotion during the reward induction leads to a faster return to baseline after the reward induction is over. This finding may also be an indication of psychological flexibility, or the ability to shift and adapt to situational demands (e.g., Kashdan & Rottenberg, 2010 ). Specifically, those individuals who were able to most fully experience and possibly savor the positive emotional state during the reward-induction also were best able to flexibly recover and detach from the experience after it was over when no other emotion was induced.
In the positive condition, higher subjective state positive emotion during the reward induction led to slower physiological recovery. Participants in this condition experienced a positive reward-salient induction immediately followed by a second positive mood induction with no need to detach, shift, or recover from the positive emotional experience. It should be considered that it may be adaptive to maintain and coast on the positive emotional and physiologically activated state when experiencing two individual positive emotion inductions. In fact, although reward sensitivity is often characterized as maladaptive given its association with risky behaviors and onset of psychopathology, Casey (2013) reminds us that characterizing something that is part of normative development (heightened reward sensitivity) as maladaptive is ill-informed. Elevated reward sensitivity may help older adolescents engage in goal-directed behaviors such as seeking out new relationships, interests, and academic pursuits. Moreover, increased neurobiological reactivity to prosocial reward is associated with prospective decreases in risk-taking behaviors ( Telzer, Fuligni, Lieberman, & Galván, 2013 ). Reward sensitivity may not be something that necessarily always needs to be “regulated,” but instead, simply finding ways for older adolescents to channel this heightened reward sensitivity into more adaptive behaviors (such as sports, extracurricular activities, or academic/career pursuits) may be a more useful route. Research examining recovery from heightened reward sensitivity states is understudied, and future work should aim to understand when recovery from reward sensitivity is adaptive or when it might relate to onset of psychopathology.
Findings from the present study should be interpreted with the confines of several limitations. First, although the reward-induction appeared to influence a reward sensitive state, because there is no known subjective way to measure heightened reward sensitivity and we did not specifically assess whether we induced heightened approach-motivation, the induction might simply be capturing elevated positive emotional arousal. Although understanding recovery from positive emotional states is important, the current study aimed to specifically assess recovery from approach-motivated and reward-sensitive positive emotional states that have been linked with sensation-seeking behaviors and negative outcomes in adolescence ( Galvan, 2013 ). Related, we used a relatively small monetary reward in addition to a social reward and thus we are unable to disentangle what participants specifically found rewarding about this task. Future studies might employ neuroimaging or electroencephalography (EEG) methodology to assess whether emotional and motivational regions implicated in reward sensitivity and approach-motivation are activated in response to this reward induction. Moreover, future research would benefit by assessing both subjectively and physiologically what aspects of the reward manipulation appear to be most rewarding. Second, the current sample size was relatively small and may have been statistically underpowered to detect observable differences. Future studies replicating these findings in larger samples are warranted. Third, the current study only utilized females. Males exhibit heightened reward and sensation seeking compared with females ( Steinberg et al., 2008 ) and future research would benefit from examining gender differences in recovering from reward sensitivity. Fourth, the current study recruited only late adolescents and emerging adults aged 18 – 21, however, reward sensitivity peaks during younger adolescence (i.e., age 14 – 15; Steinberg et al., 2008 ). Although brain development continues into the twenties as do risky behaviors and onset of psychopathology associated with reward sensitivity, using a younger and more reward-sensitive age might yield a different pattern of results. Future research would benefit from assessing recovery from reward sensitivity in younger ages and across adolescent developmental trajectories into emerging adulthood to understand how recovery from heightened reward sensitive states differs across development. Fifth, the positive emotion used to assess recovery specifically induced amusement. Although this emotion has previously been used to test the undoing hypothesis ( Fredrickson, Mancuso, et al., 2000 ), and can be conceptualized as a low approach-motivated positive emotion ( Gilbert, 2012 ; Harmon-Jones & Gable, 2009 ) amusement has demonstrated mixed effects on physiological reactivity ( Kreibig, 2010 ). Amusement might not have been the most effective emotion manipulation to aid in recovery especially considering it elevated subjective arousal in the current study, and future research should assess other low-approach motivated positive emotions, such as contentment or gratitude, to assess recovery from heightened reward sensitive positive emotional states. Sixth, the current study assessed recovery in a manner that is dependent on the standard deviation of baseline physiological reactivity. Given that the standard deviation defines the size of the confidence interval, fluctuations in physiological baseline measurements largely influence the calculation of recovery duration. Although this measurement of recovery has previously been used in foundational studies of the undoing hypothesis ( Fredrickson & Levenson, 1998 ; Fredrickson, et al., 2000 ; Tugade & Fredrickson, 2004 ) other measures of recovery might lead to different conclusions.
In light of these limitations, the current study successfully induced a heightened positive state of reward sensitivity using a novel induction and also provided the first test of the undoing hypothesis from heightened reward sensitivity in older adolescents. Results found that induced positive emotion maintained physiological arousal while state positive emotion during the reward induction was associated with faster undoing. This study provides a first step at examining ways individuals can ‘undo’ heightened reward sensitivity states. Future research would benefit from prospectively examining the long-term benefits, or dysregulation, that different types of reward reactivity and reward recovery may lead to and how this might relate to onset of risky behaviors and psychopathology.
- Allen JJB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparision of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bernardi L, Porta C, Gabutti A, Spicuzza L, Sleight P. Modulatory effects of respiration. Autonomic Neuroscience: Basic and Clinical. 2001;90:47–56. doi: 10.1016/s1566-0702(01)00267-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bernston G, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psycho-physiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavioral Therapy and Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- Brenner SL, Beauchaine TP, Sylvers PD. A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology. 2005;42(1):108–115. doi: 10.1111/j.1469-8986.2005.00261.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Casey BJ. The teenage brain: An overview. Current Directions in Psychological Science. 2013;22(2):80–81. doi: 10.1177/0963721413486971. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Casey BJ, Caudle K. The teenage brain: Self control. Current Directions in Psychological Science. 2013;22(2):82–87. doi: 10.1177/0963721413480170. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46(1):193–207. doi: 10.1037/a0016128. [ DOI ] [ PubMed ] [ Google Scholar ]
- Curby KM, Johnson KJ, Tyson A. Face to face with emotion: Holistic face processing is modulated by emotional state. Cognition & Emotion. 2012;26(1):93–102. doi: 10.1080/02699931.2011.555752. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21(01):1. doi: 10.1017/s0954579409000017. [ DOI ] [ PubMed ] [ Google Scholar ]
- Davidson RJ. Comment: Affective chronometry has come of age. Emotion Review. 2015:1–3. doi: 10.1177/1754073915590844. 1754073915590844. [ DOI ] [ Google Scholar ]
- Eaton LK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, et al. Youth risk behavior surveillance-United States, 2007, surveillance summaries. Morbidity and Mortality Weekly Report. 2008;57(SS04):1–131. [ PubMed ] [ Google Scholar ]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(03):299. doi: 10.1017/s0033291705005891. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55(6):647–654. doi: 10.1037//0003-066x.55.6.647. [ DOI ] [ PubMed ] [ Google Scholar ]
- Forbes E, Hariri AR, Martin SL, Moyles DL, Fisher PM, Brown SM, … Dahl R. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition & Emotion. 1998;12(2):191–220. doi: 10.1080/026999398379718. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motivation and Emotion. 2000;24(4):237–258. doi: 10.1023/A:1010796329158. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fredrickson BL, Maynard KE, Helms MJ, Haney TL, Siegler IC, Barefoot JC. Hostility predicts magnitude and duration of blood pressure response to anger. Jounral of Behavioral Medicine. 2000;23(3):2000. doi: 10.1023/A:1005596208324. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gable PA, Harmon-Jones E. The blues broaden, but the nasty narrows: Attentional consequences of negative affects low and high in motivational intensity. Psychological Science. 2010;21(2):211–215. doi: 10.1177/0956797609359622. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gable PA, Harmon-Jones E. Attentional consequences of pregoal and postgoal positive affects. Emotion. 2011;11(6):1358–1367. doi: 10.1037/a0025611. [ DOI ] [ PubMed ] [ Google Scholar ]
- Galambos N, Leadbeater B, Barker E. Gender differences in and risk factors for depression in adolescence: A 4-year longitudinal study. International Journal of Behavioral Development. 2004;28(1):16–25. doi: 10.1080/01650250344000235. [ DOI ] [ Google Scholar ]
- Galvan A. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/jneurosci.1062-06.2006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Galvan A. The teenage brain: Sensitivity to rewards. Current Directions in Psychological Science. 2013;22(2):88–93. doi: 10.1177/0963721413480859. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. [Comparative Study] Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gilbert KE. The neglected role of positive emotion in adolescent psychopathology. Clinical Psychology Review. 2012;32(6):467–481. doi: 10.1016/j.cpr.2012.05.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition & Emotion. 1995;9(1):87–108. doi: 10.1080/02699939508408966. [ DOI ] [ Google Scholar ]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Häfner H, Riecher A, Maurer K, Löffler W, Munk-Jørgensen P, Strömgren E. How does gender influence age at first hospitalization for schizophrenia? A transnational case register study. Psychological Medicine. 1989;19(04):903–918. doi: 10.1017/S0033291700005626. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harmon-Jones E, Gable PA. Incorporating motivational intensity and direction into the study of emotions: Implications for brain mechanisms of emotion and cognition-emotion interactions. Netherlands Journal of Psychology. 2009;64:132–142. doi: 10.1007/BF03076416. [ DOI ] [ Google Scholar ]
- Haynes SN, Gannon LR, Orimoto L, O’Brien WH, Brandt M. Psychophysiological assessment of poststress recovery. Psychological Assessment: A Journal of Consulting & Clinical Psychology A Journal of Consulting and Clinical Psychology. 1991;3(3):356–365. doi: 10.1037//1040-3590.3.3.356. [ DOI ] [ Google Scholar ]
- Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review. 2010;30(7):865–878. doi: 10.1016/j.cpr.2010.03.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;(6006):932. doi: 10.1126/science.1192439. [ DOI ] [ PubMed ] [ Google Scholar ]
- Knutson B, Fong GW, Bennett SM, Adams CS, Hommer DW. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related FMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kogan A, Gruber J, Shallcross AJ, Ford BQ, Mauss IB. Too much of a good thing? Cardiac vagal tone’s non-linear relationship with well-being. Emotion. 2013;13:599–604. doi: 10.1037/a0032725. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kok BE, Fredrickson BL. Upward spirals of the heart: Autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biological Psychology. 2010 doi: 10.1016/j.biopsycho.2010.09.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kreibig SD, Gendolla GHE, Scherer KR. Psychophysiological effects of emotional responding to goal attainment. Biological Psychology. 2010;84(3):474–487. doi: 10.1016/j.biopsycho.2009.11.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- Levenson RW. Human emotion: A functional view. In: Ekman P, Davidson R, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. pp. 123–126. [ Google Scholar ]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB. Gender differences in anxiety disorders and anxiety symptoms in adolescents. Journal of Abnormal Psychology. 1998;107(1):109–117. doi: 10.1037/0021-843X.107.1.109. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lewinsohn PM, Seeley JR, Moerk KC, Striegel-Moore RH. Gender differences in eating disorder symptoms in young adults. International Journal of Eating Disorders. 2002;32(4):426–440. doi: 10.1002/eat.10103. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lovell D, Zeffirelli F., Writers . The Champ. Culver City, CA: MGM/Pathe Home Video; 1979. [ Google Scholar ]
- Luna B, Paulsen DJ, Padmanabhan A, Geier C. The teenage brain: Cognitive control and motivation. Current Directions in Psychological Science. 2013;22(2):94–100. doi: 10.1177/0963721413478416. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [ DOI ] [ PubMed ] [ Google Scholar ]
- McEwen BS. Stress, adaptation, and sisease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nielsen L, Knutson B, Carstensen LL. Affect dynamics, affective forecasting, and aging. Emotion. 2008;8(3):318–330. doi: 10.1037/1528-3542.8.3.318. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nolen-Hoeksema S, Wisco B, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9(2):265–270. doi: 10.1037/a0015383. [ DOI ] [ PubMed ] [ Google Scholar ]
- Paus T, Keshavan MS, Giedd JN. Why do many psychiatric disorders emerge during adolescence. Nature Neuroscience. 2008:947–957. doi: 10.1038/nrn2513. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage. 2010;49(4):3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rao U, Sidhartha T, Harker KR, Bidesi AS, Chen LA, Ernst M. Relationship between adolescent risk preferences on a laboratory task and behavioral measures of risk-taking. Journal of Adolescent Health. 2011;48(2):151–158. doi: 10.1016/j.jadohealth.2010.06.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Richter M, Gendolla GHE. The heart contracts to reward: Monetary incentives and preejection period. Psychophysiology. 2009;46(3):451–457. doi: 10.1111/j.1469-8986.2009.00795.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rolls ET. The Brain and Emotion. New York: Oxford University Press; 1999. [ Google Scholar ]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2(2):135–146. doi: 10.1037//1528-3542.2.2.135. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rottenberg J, Ray RD, Gross JJ. Emotion Elicitation Using Films. In: Coan JA, Allen JJB, editors. The handbook of emotion elicitation and assessment. London: Oxford University Press; 2007. [ Google Scholar ]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110(1):145–172. doi: 10.1037/0033-295x.110.1.145. [ DOI ] [ PubMed ] [ Google Scholar ]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sheppes G, Catran E, Meiran N. Reappraisal (but not distraction) is going to make you sweat: Physiological evidence for self-control effort. International Journal of Psychophysiology. 2009;71(2):91–96. doi: 10.1016/j.ijpsycho.2008.06.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Van Doornen LJP. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Somerville LH. The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22(2):121–127. doi: 10.1177/0963721413476512. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010 doi: 10.1002/dev.20445. [ DOI ] [ PubMed ] [ Google Scholar ]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [ DOI ] [ PubMed ] [ Google Scholar ]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18(3):650–659. doi: 10.1016/S1053-8119(02)00051-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tsai J. Ideal affect: Cultural causes and behavioral consequences. Perspectives on Psychological Science. 2007;2:242–259. doi: 10.1111/j.1745-6916.2007.00043.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86(2):320–333. doi: 10.1037/0022-3514.86.2.320. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vaidya JG, Knutson B, O’Leary DS, Block RI, Magnotta V. Neural sensitivity to absolute and relative anticipated reward in adolescents. PLoS ONE. 2013;8(3):e58708. doi: 10.1371/journal.pone.0058708. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Waugh CE, Muhtadie L, Thompson RJ, Joormann J, Gotlib IH. Affective and physiological responses to stress in girls at elevated risk for depression. Development and Psychopathology. 2012;24(02):661–675. doi: 10.1017/s095457941200023. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (561.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Undoing Hypothesis of Positive Emotions

Undoing Hypothesis: How Positive Emotions Counteract Stress
Strong emotions, such as anger and fear, coincides with the activation of the sympathetic nervous system , motivating adaptive survival activity. Along with anger and fear, our heart rates increase, our blood pressure rises, glycogenolysis (breaking down glucose to create immediate energy) ensues, gastrointestinal peristalsis (a normal digestive function) ceases. When the sympathetic nervous system is activated, almost every living tissue of our body is awakened and prepared for action. In Barbara Fredrickson’s Undoing Effect theory, she proposes that positive emotions counteract the arousal, bringing the body back into healthy functioning states.
Activation of the sympathetic nervous system is the “nonspecific response of the body to any demand” ( Tan & Yip, 2018 ). Hans Selye, known for his work on stress, wrote that “stress is an interaction between damage and defense.” He explains that the physiological response to stress has “toxic effects” ( Selye, 1951 ). Physiological arousal to combat threats to our wellbeing is necessary for survival but comes at a cost.
Key Definition:
The undoing hypothesis suggests that positive emotions undo some of the damaging effects of stress and heightened arousal, bringing the body back to a healthy functioning state.
Some researchers theorize that certain positive emotions play an essential role in “undoing” the physiological arousal caused by negative emotions. One of the functions, they suggest, of positive emotions “is to facilitate tension reduction” ( Yuan, et al., 2010 ).
Silvan Solomon Tompkins (1911-1991), in reference to the stress-response, referred to “the-smiling-joy response” as a reaction to the decreases in the density of stimulation, or neural arousal, that accompanies strong emotion ( Tompkins, 2010 ). According to Tompkins, the reduction of stress causes the smile.
The undoing effect theory suggests that the smile relieves the tension. In other words, positive emotions put the brakes on the sympathetic nervous system by hastening a return to homeostasis by “undoing, or down-regulating, of physiological arousal caused by negative emotions” ( Tompkins, 2010. p. 467 ).
See Positive Emotions for more on this core topic
Health and Positive Emotions
The undoing effect is a subcategory of research that falls under a much larger, and almost universally accepted theory—positive emotions promote health. Evidence strongly suggests that positive affect (experiencing positive emotions) is associated with “a reduced risk of developing cardiovascular disease and is protective against the progression of cardiovascular disease” ( Cavanagh & Larkin, 2018 ).
Positive emotions have been theorized to promote healthy functioning that spans across social, cognitive, and physiological domains. Barbara Fredrickson theorizes that positive emotions open up new paths for development in her Broaden and Build theory . She explains, ” these broadening mindsets carry indirect and long-term adaptive benefits because broadening builds enduring personal resources, which function as reserves to be drawn upon later to manage future threats” ( Fredrickson, 2001 ).
In explaining the undoing effect, Fredrickson wrote, “If negative emotions narrow the momentary thought–action repertoire and positive emotions broaden this same repertoire, then positive emotions ought to function as efficient antidotes for the lingering effects of negative emotions” ( Fredrickson, 2001 ).
T. Franklin Murphy wrote, “the broadening of attention and stockpiling of resources contribute to wellbeing by improving future problem solving. We gather wisdom, build skills, and actively recover from demands” ( Murphy, 2020 ).
Positive emotions is postulated to impact wellness across many plains and thus researchers are curious to uncovering the mechanisms behind positive emotions that may create the conditions that lead to health and wellness. Undoing effect is one of the theories seeking to explain why positive emotions improve health and promote overall wellness.
See Positive Emotions for more on this topic
History of Undoing Hypothesis Effect of Positive Emotions
As early as 1988, Robert W. Levenson suggested that “the evolutionary meaning of positive emotions such as happiness might be to function as efficient ‘undoers’ of states of ANS arousal produced by certain negative emotions” ( Levenson, 1988, p. 23 ).
The actual theory of undoing affect can be traced back to a 1998 publication of research conducted by Barbara Fredrickson and Levenson published in the journal of Cognition and Emotion.
Fredrickson and Levenson present the undoing effect theory as: In there 1998 paper they presented two studies that utilized movies to stimulate the sympathetic nervous system response (fear and sadness) and then measured recovery time, assessing the impact of positive emotions on the speed of recovery. Fredrickson and Levenson’s findings supported the undoing effects hypothesis.
Action Tendencies and Emotion
An underlying concept essential to the undoing effect hypothesis is that negative affect evokes action tendencies. “Negative emotions that create urges for specific action requiring substantial physical energy (e.g., attack, flee) also produce heightened cardiovascular reactivity that redistributes blood flow to relevant skeletal muscles” ( Fredrickson, et al., 2000 ).
Action potentials typically focus on an environmental threat that demands specific behaviors to eliminate the threat. Our emotional responses, hence, are survival mechanisms to combat specific threats.
Positive emotions, on the other hand, are “often characterized by relative lack of autonomic reactivity” ( Fredrickson, et al., 2000, p. 238 ). The undoing effect hypothesis explains the survival benefits of positive emotions. Since they don’t elicit specific behavior through activation of the autonomic nervous system, what exactly do they do?
According to Fredrickson and Levenson, positive emotions have two primary purposes:
- First is the broadening effect. The broadening effect suggests that during positive emotions we gather enduring resources that contribute to resilience during more trying times.
- Second is the undoing effect. The undoing effect suggests that positive emotion states reset the body, bringing it back into a homeostatic balance.
Positive Emotions and Negative Emotions
In contrast to negative emotions, which promote survival in the moment by addressing specific threats with life preserving actions, positive emotions promote survival over the long run by building resources that could be drawn upon later. Positive emotions “can build a variety of enduring personal resources” ( Fredrickson, et al., 2000, p. 239 ). The second purpose is the regulating influence positive emotions have on arousal, down regulating the autonomic nervous system, returning to a physiological homeostasis.
Negative affect floods the biological system with activating chemicals that evolved to prepare the body for threat. However, a prolonged, overabundance of those chemicals damages organs, leading do illness and disease. Murphy explains, “these physical changes have a cost, and when that cost exceeds our ability to process we become vulnerable to predispositioned diseases” ( Murphy, 2021 ).
Selye explained that “the effects of stress depend not only on the magnitude and duration of the stressor, but also on the strategies individuals adopt to cope with it” ( Heller & LaPierre, 2012, Kindle location: 1,772 ).
The undoing effect is an unconscious mechanism that the body uses to reset biological levels by down regulating arousal through positive emotions.
Empirical Support for Undoing Effect
Early studies found substantial support for Levenson and Fredrickson’s undoing hypothesis . However , more recent studies throw some doubt on the theory. While positive emotions are empirically supported to be associated with health, the undoing effect may not be the cause for the association.
Many of the terms used in the early studies were broad and difficult to quantify. When researchers accounted for other confounding variables (resiliency, flourishing, appraisal style, etc..) only a few of the previous cited studies supported the undoing hypothesis. Most of the studies. “It is possible that these individual characteristics exert a more powerful effect on physiological recovery than state positive affect, which may explain why several studies either found partial support or failed to support the undoing hypothesis altogether” ( Cavanagh & Larkin, 2018 ).
In a 2022 meta-analytic review of whether positive emotions facilitate autonomic nervous system recovery, the authors “found no support for the general undoing effect of positive emotions” ( Behnke, et al., p. 14 ).
Related Concepts
The undoing effect of positive emotions is a fascinating area within psychology that suggests positive emotions can neutralize the lingering effects of negative emotions. Here are some related concepts and theories:
- Broaden-and-Build Theory : This theory, proposed by Barbara Fredrickson, suggests that positive emotions broaden an individual’s momentary thought-action repertoire, which in turn builds their enduring personal resources, ranging from physical and intellectual resources to social and psychological ones.
- Resilience Theory : Resilience in psychology refers to the ability to cope with and bounce back from adversity. Positive emotions play a crucial role in resilience, helping individuals recover from stress and trauma.
- Emotion Regulation : This concept involves strategies people use to influence their own emotional experience. The undoing effect can be seen as a form of emotion regulation, where positive emotions help to down-regulate the negative emotions.
- Affective-Cognitive Consistency : This theory posits that when individuals experience positive emotions, their cognitive processes are more likely to be congruent with their affective states, leading to a more harmonious internal state.
- Positive Psychology Interventions : These interventions aim to increase well-being and happiness, often by leveraging the undoing effect to counterbalance negative emotions and enhance positive ones.
- Stress Recovery Theory : This theory suggests that positive emotions facilitate the recovery from the physiological effects of stress, aligning closely with the undoing effect.
- Opponent-Process Theory : Originally a theory of color vision, this has been applied to emotions as well, suggesting that an emotional experience can trigger its opposite, to restore emotional balance.
These theories and concepts all contribute to our understanding of how positive emotions can counteract negative ones and promote psychological well-being and health. If you’re interested in exploring these concepts further, I can provide more detailed explanations or resources.
A Few Final Words On the Undoing Effect
Research still supports the broader category of positive emotions and health. Whether positive emotions are a cause or symptom to health is still under investigation. Perhaps, there is an undoing effect. It just isn’t the sole cause for or purpose of positive emotion.
We know some protect against intense emotions with escape through “gallows humor ,” or a healthy laugh while in the depths of sorrow. Somehow these mechanisms protect against realities that overwhelm our threshold to process, and potentially causing damage to our bodies. Certainly, we should continue to promote positive emotions even if we aren’t completely certain of the process behind them. Undoubtedly, resetting our bodies to healthier states will create better health and promote a greater senses of wellness.
Last Update: December 18, 2024
Type your email…
References:
Bahrami, F., Kasaei, R., & Zamani, A. ( 2012 ). Preventing Worry and Rumination by Induced Positive Emotion. International Journal of Preventive Medicine , 3(2), 102-109.
Behnke, M., Pietruch, M., Chwiłkowska, P., Wessel, E., Kaczmarek, L., Assink, M., & Gross, J. ( 2022 ). The Undoing Effect of Positive Emotions: A Meta-Analytic Review. Emotion Review,OnlineFirst, 1. DOI: 10.1177/17540739221104457
Cavanagh, C., & Larkin, K. ( 2018 ). A Critical Review of the “Undoing Hypothesis”: Do Positive Emotions Undo the Effects of Stress?. Applied Psychophysiology and Biofeedback , 43(4), 259-273. DOI: 10.1007/s10484-018-9412-6
Fredrickson, B. L., & Levenson, R. ( 1998 ). Positive Emotions Speed Recovery from the Cardiovascular Sequelae of Negative Emotions. Cognition & Emotion , 12(2), 191-220. DOI: 10.1080/026999398379718
Fredrickson, B. L., Mancuso, R. A., Branigan, C. and Tugade, M. M. ( 2000 ). The undoing effect of positive emotions. Motivation and Emotion 24, 237–258. DOI: 10.1023/A:1010796329158
Fredrickson, B. ( 2001 ). The role of positive emotions in positive Psychology. The broaden-and build theory of positive emotions. The American psychologist , 56(3), 218-226. DOI: 10.1037/0003-066X.56.3.218
Spotlight Book:
Heller, Lawrence; LaPierre, Aline ( 2012 ). Healing Developmental Trauma: How Early Trauma Affects Self-Regulation, Self-Image, and the Capacity for Relationship. North Atlantic Books ; 1st edition.
Levenson, R. W. ( 1988 ). Emotion and the autonomic nervous system: A prospectus for research on autonomic specificity. In H. L. Wagner (Ed.), Social psychophysiology and emotion: Theory and clinical applications (pp. 17–42). John Wiley & Sons.
Murphy, T. Franklin ( 2020 ). Broaden and Build Theory. Psychology Fanatic. Published: 9-4-2020. Accessed: 9-24-2022.
Murphy, T. Franklin ( 2021 ) Diathesis Stress Model. Psychology Fanatic . Published: 9-7-2021. Accessed: 9-25-2022.
Selye, H. ( 1951 ). The General-Adaptation-Syndrome. Annual Review of Medicine , 2(1), 327-342.
Tan, Siang Yong, Yip, A ( 2018 ) Hans Selye (1907–1982): Founder of the stress theory.
Yuan, J., McCarthy, M., Holley, S., & Levenson, R. ( 2010 ). Physiological Down-Regulation and Positive Emotion in Marital Interaction. Emotion , 10(4), 467-474. DOI: 10.1037/a0018699
Resources and Articles
Please visit Psychology Fanatic’s vast selection of articles , definitions and database of referenced books .
* Many of the quotes from books come books I have read cover to cover. I created an extensive library of notes from these books. I make reference to these books when using them to support or add to an article topic. Most of these books I read on a kindle reader. The Kindle location references seen through Psychology Fanatic is how kindle notes saves my highlights.
Please note that some of the links in this article are affiliate links. If you purchase a product through one of these links, I may receive a small commission at no additional cost to you. This helps support the creation of high-quality content and allows me to continue providing valuable information.
The peer reviewed article references mostly come from Deepdyve . This is the periodical database that I have subscribe to for nearly a decade. Over the last couple of years, I have added a DOI reference to cited articles for the reader’s convenience and reference.
Thank-you for visiting Psychology Fanatic. Psychology Fanatic represents nearly two decades of work, research, and passion.
Topic Specific Databases:
PSYCHOLOGY – EMOTIONS – RELATIONSHIPS – WELLNESS – PSYCHOLOGY TOPICS
Disclaimer:
The information provided in this blog is for general informational purposes only and does not constitute medical advice. It is essential to consult with a qualified healthcare professional for any health concerns or before making any significant changes to your lifestyle or treatment plan.
Share this:
About the author.
T. Franklin Murphy
Related posts.

Hope Theory
Hope theory, developed by C. Richard Snyder, emphasizes the role of hope in motivation and goal pursuit. It involves belief in finding pathways to achieve…
Read More »

Positive Thinking Mantras
Rhymes, quotes and mental health mantras have a purpose. They lift our spirits and motivate action. Alone, however, they are not enough.

Humor: A Defense Mechanism
Humor serves as a healthy defense mechanism, offering individuals a valuable means to cope with challenging situations, alleviate tension, and foster social bonds. It provides…

Psychology of Awe
Awe has a great psychological impact and transformative potential. It explores the perception of vastness, spiritual experiences, and the impact of awe on prosocial behaviors.…

Negative Self Talk
The harmful effects of negative self-talk can be crippling, leading to low self-esteem and mental health issues. By challenging and transforming this inner dialogue, individuals…

Positive Psychology
Positive psychology is a branch of psychology focusing on the study and promotion of strengths, virtues, and positive emotions to enhance well-being. Main topics include…
Leave a Reply Cancel reply
Discover more from psychology fanatic.
Subscribe now to keep reading and get access to the full archive.
Continue reading

IMAGES
COMMENTS
Sep 14, 2024 · Undoing in Action: Examples from Everyday Life. To truly grasp the concept of undoing, it’s helpful to see how it plays out in real-life scenarios. Undoing is not confined to the therapist’s office or psychology textbooks; it’s a phenomenon that occurs in our daily lives, often without us even realizing it.
Undoing is a defense mechanism in which a person tries to cancel out or remove an unhealthy, destructive or otherwise threatening thought or action by engaging in contrary behavior. For example, after thinking about being violent with someone, one would then be overly nice or accommodating to them.
Two samples of university students were tested. Each provides an independent test of the undoing hypothesis. Sample 1 included 95 university students (50% women) recruited for a study on emotions through flyers and newspaper advertisements. Each was paid $30 to participate in a series of studies lasting 2 hr.
Jun 6, 2022 · Since this undoing hypothesis was first proposed, dozens of studies have sought to test this hypothesis, but findings to date have been mixed (Cavanagh & Larkin, 2018).In the present review, we employ a meta-analytic technique to quantitatively synthesize the literature on the undoing effect of positive emotions, examining the relationship between positive emotions and autonomic nervous system ...
In a study that tested the undoing effect (Fredrickson et al., 2000), participants first completed baseline measures of heart rate, finger pulse, and blood pressure. Then, all participants were induced to feel a high-arousal negative emotion by telling participants they would have 60 seconds to write a 3-minute speech on a topic provided to them.
Given that adults can effectively use low-approach positive emotional states to ‘undo’ physiological stress and arousal (Fredrickson, et al., 2000), the first undoing hypothesis predicted that low approach-motivated positive emotion would facilitate effective emotion recovery (i.e., decrease in emotional and physiological intensity via ...
Notably, the undoing hypothesis suggests a novel relationship between pos-itive emotions and cardiovascular reactivity. Perhaps positive emotions do not themselves generate cardiovascular reactivity, but instead quell any existing car-diovascular reactivity caused by negative emotions. Put differently, a prior state
Sep 4, 2020 · The undoing hypothesis suggests that positive emotions undo some of the damaging effects of stress and heightened arousal, bringing the body back to a healthy functioning state. Some researchers theorize that certain positive emotions play an essential role in “undoing” the physiological arousal caused by negative emotions.
Sep 1, 2012 · The undoing hypothesis was also tested using longitudinal study designs in real-life contexts. For example, a recent experience sampling study conducted on workers measured positive and negative affect at work for two consecutive weeks and job satisfaction at the end.
For example, depression and anxiety are both associated with increased negative ... Frederickson’s (1998) undoing hypothesis argues that positive affect protects an individual’s physical ...